当前位置:
X-MOL 学术
›
Adv. Healthcare Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH-Sensitive Molecular-Switch-Containing Polymer Nanoparticle for Breast Cancer Therapy with Ferritinophagy-Cascade Ferroptosis and Tumor Immune Activation
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2021-09-17 , DOI: 10.1002/adhm.202100683 Tiantian Zuo 1 , Tianxu Fang 1 , Jun Zhang 1 , Jie Yang 1 , Rui Xu 1 , Zhihua Wang 1 , Huizi Deng 1 , Qi Shen 1
Advanced Healthcare Materials ( IF 10.0 ) Pub Date : 2021-09-17 , DOI: 10.1002/adhm.202100683 Tiantian Zuo 1 , Tianxu Fang 1 , Jun Zhang 1 , Jie Yang 1 , Rui Xu 1 , Zhihua Wang 1 , Huizi Deng 1 , Qi Shen 1
Affiliation
|
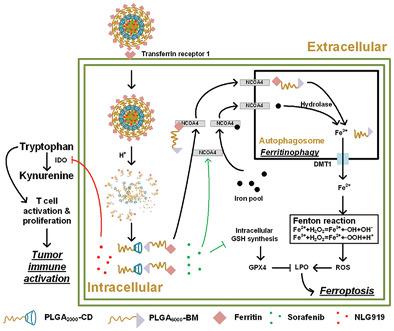
Ferritin internalized into tumor cells is degraded and releases iron ions via ferritinophagy. Iron ions participate in Fenton reaction to produce reactive oxygen species for lipid peroxidation and ferroptosis. Inhibition of indoleamine-2,3-dioxygenase (IDO) decreases tryptophan elimination to induce T cells activation for tumor immunosuppression relief. The active tumor targeting nanoparticles containing ferritin and a pH-sensitive molecular-switch (FPBC@SN) are developed to utilize ferritinophagy-cascade ferroptosis and tumor immunity activation for cancer therapy. FPBC@SN disintegrates in acidic cytoplasm and releases sorafenib (SRF) and IDO inhibitor (NLG919). SRF upregulates nuclear receptor coactivator 4 (NCOA4) to induce ferritin and endogenous iron pool degradation by ferritinophagy, then obtained iron ions participate in the Fenton reaction to produce lipid peroxide (LPO). Meanwhile, SRF blocks glutathione synthesis to downregulate glutathione peroxidase 4 (GPX4) which can scavenge LPO as a different pathway from ferritinophagy to promote ferroptosis in tumor cells. NLG919 inhibits IDO to reduce tryptophan metabolism, so immunity in tumors is aroused to anti-tumor. In vitro and in vivo experiments prove FPBC@SN inhibits tumor cell growth and metastasis, indicating the potential of FPBC@SN for breast cancer therapy based on the combination of ferritinophagy-cascade ferroptosis and tumor immunity activation.
中文翻译:

用于乳腺癌治疗的 pH 敏感分子开关聚合物纳米颗粒
内化到肿瘤细胞中的铁蛋白被降解并通过铁蛋白吞噬作用释放铁离子。铁离子参与芬顿反应产生活性氧,用于脂质过氧化和铁死亡。抑制吲哚胺-2,3-双加氧酶 (IDO) 会减少色氨酸的消除,从而诱导 T 细胞活化以缓解肿瘤免疫抑制。开发了含有铁蛋白和 pH 敏感分子开关 (FPBC@SN) 的活性肿瘤靶向纳米颗粒,以利用铁蛋白吞噬-级联铁死亡和肿瘤免疫激活进行癌症治疗。FPBC@SN 在酸性细胞质中分解并释放索拉非尼 (SRF) 和 IDO 抑制剂 (NLG919)。SRF 上调核受体共激活因子 4 (NCOA4) 以通过铁蛋白吞噬诱导铁蛋白和内源性铁池降解,然后得到的铁离子参与芬顿反应生成脂质过氧化物(LPO)。同时,SRF 阻断谷胱甘肽合成以下调谷胱甘肽过氧化物酶 4 (GPX4),该酶可以清除 LPO,这是一种不同于铁蛋白吞噬的途径,以促进肿瘤细胞中的铁死亡。NLG919通过抑制IDO降低色氨酸代谢,从而激发肿瘤免疫抗肿瘤。体外和体内实验证明 FPBC@SN 抑制肿瘤细胞的生长和转移,表明 FPBC@SN 基于铁蛋白吞噬-级联铁死亡和肿瘤免疫激活相结合的乳腺癌治疗潜力。SRF 阻断谷胱甘肽合成以下调谷胱甘肽过氧化物酶 4 (GPX4),它可以清除 LPO,这是一种不同于铁蛋白吞噬的途径,以促进肿瘤细胞中的铁死亡。NLG919通过抑制IDO降低色氨酸代谢,从而激发肿瘤免疫抗肿瘤。体外和体内实验证明 FPBC@SN 抑制肿瘤细胞的生长和转移,表明 FPBC@SN 基于铁蛋白吞噬-级联铁死亡和肿瘤免疫激活相结合的乳腺癌治疗潜力。SRF 阻断谷胱甘肽合成以下调谷胱甘肽过氧化物酶 4 (GPX4),它可以清除 LPO,这是一种不同于铁蛋白吞噬的途径,以促进肿瘤细胞中的铁死亡。NLG919通过抑制IDO降低色氨酸代谢,从而激发肿瘤免疫抗肿瘤。体外和体内实验证明 FPBC@SN 抑制肿瘤细胞的生长和转移,表明 FPBC@SN 基于铁蛋白吞噬-级联铁死亡和肿瘤免疫激活相结合的乳腺癌治疗潜力。
更新日期:2021-11-04
中文翻译:

用于乳腺癌治疗的 pH 敏感分子开关聚合物纳米颗粒
内化到肿瘤细胞中的铁蛋白被降解并通过铁蛋白吞噬作用释放铁离子。铁离子参与芬顿反应产生活性氧,用于脂质过氧化和铁死亡。抑制吲哚胺-2,3-双加氧酶 (IDO) 会减少色氨酸的消除,从而诱导 T 细胞活化以缓解肿瘤免疫抑制。开发了含有铁蛋白和 pH 敏感分子开关 (FPBC@SN) 的活性肿瘤靶向纳米颗粒,以利用铁蛋白吞噬-级联铁死亡和肿瘤免疫激活进行癌症治疗。FPBC@SN 在酸性细胞质中分解并释放索拉非尼 (SRF) 和 IDO 抑制剂 (NLG919)。SRF 上调核受体共激活因子 4 (NCOA4) 以通过铁蛋白吞噬诱导铁蛋白和内源性铁池降解,然后得到的铁离子参与芬顿反应生成脂质过氧化物(LPO)。同时,SRF 阻断谷胱甘肽合成以下调谷胱甘肽过氧化物酶 4 (GPX4),该酶可以清除 LPO,这是一种不同于铁蛋白吞噬的途径,以促进肿瘤细胞中的铁死亡。NLG919通过抑制IDO降低色氨酸代谢,从而激发肿瘤免疫抗肿瘤。体外和体内实验证明 FPBC@SN 抑制肿瘤细胞的生长和转移,表明 FPBC@SN 基于铁蛋白吞噬-级联铁死亡和肿瘤免疫激活相结合的乳腺癌治疗潜力。SRF 阻断谷胱甘肽合成以下调谷胱甘肽过氧化物酶 4 (GPX4),它可以清除 LPO,这是一种不同于铁蛋白吞噬的途径,以促进肿瘤细胞中的铁死亡。NLG919通过抑制IDO降低色氨酸代谢,从而激发肿瘤免疫抗肿瘤。体外和体内实验证明 FPBC@SN 抑制肿瘤细胞的生长和转移,表明 FPBC@SN 基于铁蛋白吞噬-级联铁死亡和肿瘤免疫激活相结合的乳腺癌治疗潜力。SRF 阻断谷胱甘肽合成以下调谷胱甘肽过氧化物酶 4 (GPX4),它可以清除 LPO,这是一种不同于铁蛋白吞噬的途径,以促进肿瘤细胞中的铁死亡。NLG919通过抑制IDO降低色氨酸代谢,从而激发肿瘤免疫抗肿瘤。体外和体内实验证明 FPBC@SN 抑制肿瘤细胞的生长和转移,表明 FPBC@SN 基于铁蛋白吞噬-级联铁死亡和肿瘤免疫激活相结合的乳腺癌治疗潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号