Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2021-09-17 , DOI: 10.1016/j.apsb.2021.09.011 Zhihao Fu 1 , Hongchuan Liu 2 , Lan Wang 1 , Chuanfei Yu 1 , Yalan Yang 1 , Meiqing Feng 2 , Junzhi Wang 1
|
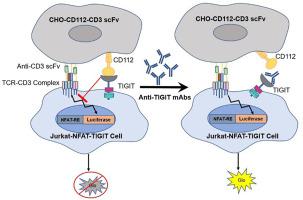
T cell immunoglobulin and ITIM domain (TIGIT) is a novel immune checkpoint that has been considered as a target in cancer immunotherapy. Current available bioassays for measuring the biological activity of therapeutic antibodies targeting TIGIT are restricted to mechanistic investigations because donor primary T cells are highly variable. Here, we designed a reporter gene assay comprising two cell lines, namely, CHO-CD112-CD3 scFv, which stably expresses CD112 (PVRL2, nectin-2) and a membrane-bound anti-CD3 single-chain fragment variable (scFv) as the target cell, and Jurkat-NFAT-TIGIT, which stably expresses TIGIT as well as the nuclear factor of activated T-cells (NFAT) response element-controlled luciferase gene, as the effector cell. The anti-CD3 scFv situated on the target cells activates Jurkat-NFAT-TIGIT cells through binding and crosslinking CD3 molecules of the effector cell, whereas interactions between CD112 and TIGIT prevent activation. The presence of anti-TIGIT mAbs disrupts their interaction, which in turn reverses the inactivation and luciferase expression. Optimization and validation studies have demonstrated that this assay is superior in terms of specificity, accuracy, linearity, and precision. In summary, this reliable and effective reporter gene assay may potentially be utilized in lot release control, stability assays, screening, and development of novel TIGIT-targeted therapeutic antibodies.
中文翻译:

用于确定靶向 TIGIT 的治疗性抗体的生物活性的报告基因分析
T 细胞免疫球蛋白和 ITIM 结构域 (TIGIT) 是一种新型免疫检查点,已被视为癌症免疫治疗的靶点。目前可用于测量靶向 TIGIT 的治疗性抗体的生物活性的生物测定仅限于机制研究,因为供体原代 T 细胞是高度可变的。在这里,我们设计了一种报告基因检测方法,包括两种细胞系,即 CHO-CD112-CD3 scFv,其稳定表达 CD112(PVRL2,nectin-2)和膜结合抗 CD3 单链片段变量(scFv)为靶细胞和作为效应细胞稳定表达TIGIT以及活化T细胞核因子(NFAT)反应元件控制的荧光素酶基因的Jurkat-NFAT-TIGIT。位于靶细胞上的抗 CD3 scFv 通过结合和交联效应细胞的 CD3 分子激活 Jurkat-NFAT-TIGIT 细胞,而 CD112 和 TIGIT 之间的相互作用阻止激活。抗 TIGIT mAb 的存在破坏了它们的相互作用,进而逆转了失活和荧光素酶的表达。优化和验证研究表明,该测定在特异性、准确性、线性和精密度方面具有优势。总之,这种可靠且有效的报告基因检测可能潜在地用于批次释放控制、稳定性检测、筛选和新型 TIGIT 靶向治疗性抗体的开发。这反过来又逆转了失活和荧光素酶的表达。优化和验证研究表明,该测定在特异性、准确性、线性和精密度方面具有优势。总之,这种可靠且有效的报告基因检测可能潜在地用于批次释放控制、稳定性检测、筛选和新型 TIGIT 靶向治疗性抗体的开发。这反过来又逆转了失活和荧光素酶的表达。优化和验证研究表明,该测定在特异性、准确性、线性和精密度方面具有优势。总之,这种可靠且有效的报告基因检测可能潜在地用于批次释放控制、稳定性检测、筛选和新型 TIGIT 靶向治疗性抗体的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号