Renewable Energy ( IF 8.7 ) Pub Date : 2021-09-17 , DOI: 10.1016/j.renene.2021.09.021 Rui Han 1, 2 , Shuang Xing 1, 2 , Xueqian Wu 1, 2 , Caihong Pang 1, 2 , Shuangchun Lu 1, 2 , Yun Su 1, 2 , Qingling Liu 1, 2 , Chunfeng Song 1, 2 , Jihui Gao 3
|
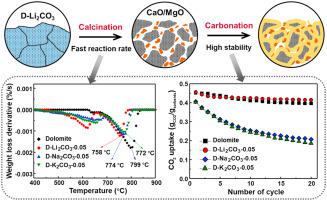
The calcium-Looping process is an advantageous candidate for thermochemical energy storage in Concentrated Solar Power plants. Achieving fast thermal energy storage at a moderate temperature would be highly beneficial for the heat storage process. In this study, commercially available alkali carbonates (Li2CO3, Na2CO3, K2CO3) were used as dopants for limestone/dolomite to improve their decarbonation rates and cycling stability. Results show that alkali carbonate doping reduces the decarbonation temperature of limestone and dolomite by accelerating the ion diffusion during decomposition. However, the doping of alkali carbonate also enhanced the sintering of limestone-based CaO so that the alkali carbonate doped limestones fail to maintain their heat storage capacity after multiple cycles. In contrast, the Li2CO3 doped dolomite still maintains excellent cyclic stability, attributed to the inhibition effect of the MgO skeleton on sintering. From these results, we then derived the effect scheme of Li2CO3 on the decarbonation and carbonation of limestone or dolomite. This study presents a simple yet effective method of reducing the heat storage temperature of Ca-based mineral materials and maintaining their cycling stability, simultaneously, with promising potential for industrial use.
中文翻译:

碱金属碳酸盐掺杂对CaO/CaCO3循环过程中Ca基天然矿物热化学储能的相关影响
钙循环过程是聚光太阳能发电厂中热化学能量存储的有利候选者。在适中的温度下实现快速的热能储存对储热过程非常有益。在本研究中,市售的碱金属碳酸盐(Li 2 CO 3、Na 2 CO 3、K 2 CO 3) 被用作石灰石/白云石的掺杂剂,以提高它们的脱碳率和循环稳定性。结果表明,碱金属碳酸盐掺杂通过加速分解过程中的离子扩散来降低石灰石和白云石的脱碳温度。然而,碱金属碳酸盐的掺杂也增强了石灰石基 CaO 的烧结,使得碱金属碳酸盐掺杂的石灰石在多次循环后无法保持其蓄热能力。相比之下,Li 2 CO 3掺杂的白云石仍然保持优异的循环稳定性,这归因于MgO骨架对烧结的抑制作用。从这些结果中,我们推导出了 Li 2 CO 3的影响方案关于石灰石或白云石的脱碳和碳化。这项研究提出了一种简单而有效的方法,可以降低钙基矿物材料的储热温度并保持其循环稳定性,同时具有良好的工业应用潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号