当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of metal centers of complexes bearing bipyridine ligand for electrochemical- and photochemical-driven hydrogen evolution
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2021-09-16 , DOI: 10.1002/aoc.6453 Chun‐Li Wang 1 , Hao Yang 1 , Juan Du 1 , Shu‐Zhong Zhan 1
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2021-09-16 , DOI: 10.1002/aoc.6453 Chun‐Li Wang 1 , Hao Yang 1 , Juan Du 1 , Shu‐Zhong Zhan 1
Affiliation
|
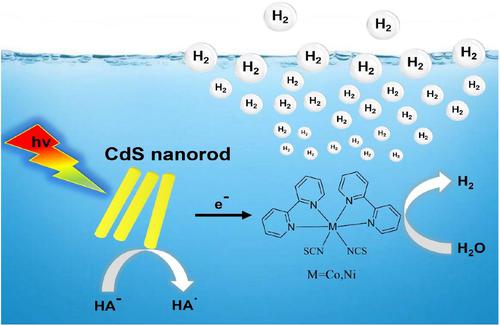
In this work, two complexes with bipyridine ligand, [Co (bpy)2(SCN)2] 1 and [Ni (bpy)2(SCN)2] 2 have been prepared and well determined by single crystal X-crystallography, power X-ray diffraction (XRD), IR spectra, and so forth. Both exhibit good catalytic activity for electrochemical- and photochemical driven-hydrogen evolution. Complexes 1 and 2 can electro-catalyze H2 evolution from a neutral buffer (pH 7.0) with a turnover frequency (TOF) of 699.6 and 1469 mol H2/mol catalyst/h at an overpotential (OP) of 837.6 mV, respectively. Under blue light (λ = 469 nm), together with CdS nanorods (CdS NRs) as a photosensitizer, and ascorbic acid as a sacrificial electron donor, complexes 1 and 2 also can photo-catalyze H2 evolution from water. Under an optimal condition, the turnover numbers (TONs) reach 10,600 (complex 1) and 15,180 (complex 2) mol of H2 per mol of catalyst during 48-h irradiation, respectively. The average apparent quantum yields (AQYs) of complex 1 and complex 2 are 15.07% and 22.7%, respectively. Electrochemical and photochemical investigations show that the nickel complex has a higher activity for hydrogen evolution than that of the cobalt complex. These results can provide a new insight into design and manufacture of new H2-production catalyst.
中文翻译:

带有联吡啶配体的配合物的金属中心对电化学和光化学驱动析氢的影响
在这项工作中,制备了两种具有联吡啶配体的配合物,[Co (bpy) 2 (SCN) 2 ] 1和 [Ni (bpy) 2 (SCN) 2 ] 2,并通过单晶 X 晶体学、功率 X射线衍射(XRD)、红外光谱等。两者都对电化学和光化学驱动的析氢表现出良好的催化活性。配合物1和2可以电催化 H 2从中性缓冲液 (pH 7.0) 中释放出来,转换频率 (TOF) 为 699.6 和 1469 mol H 2/mol 催化剂/h 分别在 837.6 mV 的过电势 (OP) 下。在蓝光 (λ = 469 nm) 下,与作为光敏剂的 CdS 纳米棒 (CdS NRs) 和作为牺牲电子供体的抗坏血酸一起,配合物1和2也可以光催化水中的 H 2析出。在最佳条件下,在 48 小时照射期间,每 mol 催化剂的周转数 (TON) 分别达到 10,600(络合物1)和 15,180(络合物2)mol H 2 。复合物1和复合物2的平均表观量子产率 (AQY)分别为 15.07% 和 22.7%。电化学和光化学研究表明,镍络合物的析氢活性高于钴络合物。这些结果可以为设计和制造新的H 2生产催化剂提供新的见解。
更新日期:2021-09-16
中文翻译:

带有联吡啶配体的配合物的金属中心对电化学和光化学驱动析氢的影响
在这项工作中,制备了两种具有联吡啶配体的配合物,[Co (bpy) 2 (SCN) 2 ] 1和 [Ni (bpy) 2 (SCN) 2 ] 2,并通过单晶 X 晶体学、功率 X射线衍射(XRD)、红外光谱等。两者都对电化学和光化学驱动的析氢表现出良好的催化活性。配合物1和2可以电催化 H 2从中性缓冲液 (pH 7.0) 中释放出来,转换频率 (TOF) 为 699.6 和 1469 mol H 2/mol 催化剂/h 分别在 837.6 mV 的过电势 (OP) 下。在蓝光 (λ = 469 nm) 下,与作为光敏剂的 CdS 纳米棒 (CdS NRs) 和作为牺牲电子供体的抗坏血酸一起,配合物1和2也可以光催化水中的 H 2析出。在最佳条件下,在 48 小时照射期间,每 mol 催化剂的周转数 (TON) 分别达到 10,600(络合物1)和 15,180(络合物2)mol H 2 。复合物1和复合物2的平均表观量子产率 (AQY)分别为 15.07% 和 22.7%。电化学和光化学研究表明,镍络合物的析氢活性高于钴络合物。这些结果可以为设计和制造新的H 2生产催化剂提供新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号