当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Catalysis of Electrochemical Reactions: Competition between Reduction of the Substrate and Deactivation of the Catalyst by a Cosubstrate Application to N2O Reduction
ChemElectroChem ( IF 3.5 ) Pub Date : 2021-09-17 , DOI: 10.1002/celc.202101064 Rana Deeba 1 , Sylvie Chardon-Noblat 1 , Cyrille Costentin 2
ChemElectroChem ( IF 3.5 ) Pub Date : 2021-09-17 , DOI: 10.1002/celc.202101064 Rana Deeba 1 , Sylvie Chardon-Noblat 1 , Cyrille Costentin 2
Affiliation
|
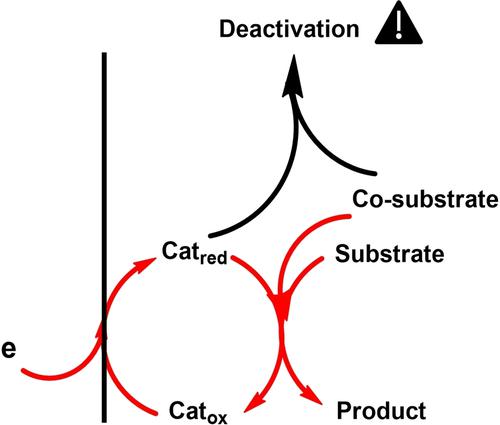
In the context of molecular catalysis of electrochemical reactions, the competition between reduction of the substrate and deactivation of the catalyst by a cosubstrate is investigated. It is a frequent situation because proton donors are ubiquitous cosubstrates in reductive electrochemical reactions and molecular catalysts, either transition metal complexes or organic aromatic molecules, and are often prone to electrohydrogenation. We provide a formal kinetic analysis in the framework of cyclic voltammetry, and we show that the response is governed by two parameters and that the competition does not depend on the scan rate. From this analysis a methodology is proposed to analyze such systems and then illustrated via the study of N2O to N2 electroreduction catalyzed by 4-cyanopyridine in acetonitrile electrolyte with water as proton donor. Incidentally, new insights into the mechanism of 4-cyanopyridine radical anion protonation are revealed.
中文翻译:

电化学反应的分子催化:通过共底物应用到 N2O 还原,底物还原和催化剂失活之间的竞争
在电化学反应的分子催化背景下,研究了底物还原与共底物对催化剂失活之间的竞争。这是一种常见的情况,因为质子供体是还原电化学反应和分子催化剂中无处不在的共底物,无论是过渡金属配合物还是有机芳香分子,并且通常容易发生电氢化。我们在循环伏安法的框架内提供了正式的动力学分析,我们表明响应受两个参数控制,并且竞争不依赖于扫描速率。根据该分析提出了一种方法来分析此类系统,然后通过对 N 2 O 到 N 2的研究加以说明4-氰基吡啶在乙腈电解质中以水为质子供体催化的电还原。顺便说一下,揭示了对 4-氰基吡啶自由基阴离子质子化机制的新见解。
更新日期:2021-10-08
中文翻译:

电化学反应的分子催化:通过共底物应用到 N2O 还原,底物还原和催化剂失活之间的竞争
在电化学反应的分子催化背景下,研究了底物还原与共底物对催化剂失活之间的竞争。这是一种常见的情况,因为质子供体是还原电化学反应和分子催化剂中无处不在的共底物,无论是过渡金属配合物还是有机芳香分子,并且通常容易发生电氢化。我们在循环伏安法的框架内提供了正式的动力学分析,我们表明响应受两个参数控制,并且竞争不依赖于扫描速率。根据该分析提出了一种方法来分析此类系统,然后通过对 N 2 O 到 N 2的研究加以说明4-氰基吡啶在乙腈电解质中以水为质子供体催化的电还原。顺便说一下,揭示了对 4-氰基吡啶自由基阴离子质子化机制的新见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号