Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2021-09-17 , DOI: 10.1016/j.bbagen.2021.130015 José L Neira 1 , Martina Palomino-Schätzlein 2 , Estefanía Hurtado-Gómez 3 , María G Ortore 4 , Alberto Falcó 3
|
Background
The phosphotransferase system (PTS) modulates the preferential use of sugars in bacteria. It is formed by a protein cascade in which the first two proteins are general (namely enzyme I, EI, and the histidine phosphocarrier protein, HPr) and the others are sugar-specific permeases; the active site of HPr is His15. The HPr kinase/phosphorylase (HPrK/P), involved in the use of carbon sources in Gram-positive, phopshorylates HPr at a serine. The regulator of sigma D protein (Rsd) also binds to HPr. We are designing specific fragments of HPr, which can be used to interfere with those protein-protein interactions (PPIs), where the intact HPr intervenes.
Methods
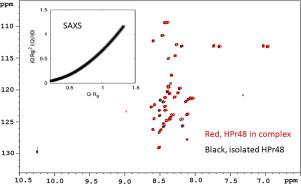
We obtained a fragment (HPr48) comprising the first forty-eight residues of HPr. HPr48 was disordered as shown by fluorescence, far-ultraviolet (UV) circular dichroism (CD), small angle X-ray scattering (SAXS) and nuclear magnetic resonance (NMR).
Results
Secondary structure propensities, from the assigned backbone nuclei, further support the unfolded nature of the fragment. However, HPr48 was capable of binding to: (i) the N-terminal region of EI, EIN; (ii) the intact Rsd; and, (iii) HPrK/P, as shown by fluorescence, far-UV CD, NMR and biolayer interferometry (BLI). The association constants for each protein, as measured by fluorescence and BLI, were in the order of the low micromolar range, similar to those measured between the intact HPr and each of the other macromolecules.
Conclusions
Although HPr48 is forty-eight-residue long, it assisted antibiotics to exert antimicrobial activity.
General significance
HPr48 could be used as a lead compound in the development of new antibiotics, or, alternatively, to improve the efficiency of existing ones.
中文翻译:

组氨酸磷酸载体蛋白 HPr 的 N 端半片段是无序的,但与 HPr 伴侣结合并显示出抗菌特性
背景
磷酸转移酶系统(PTS)调节细菌对糖的优先使用。它由蛋白质级联形成,其中前两种蛋白质是通用蛋白质(即酶 I,EI 和组氨酸磷酸载体蛋白,HPr),其他是糖特异性通透酶; HPr 的活性位点是 His15。 HPr 激酶/磷酸化酶 (HPrK/P) 参与革兰氏阳性菌碳源的利用,使 HPr 在丝氨酸处磷酸化。西格玛 D 蛋白 (Rsd) 的调节剂也与 HPr 结合。我们正在设计 HPr 的特定片段,它可用于干扰那些由完整 HPr 干预的蛋白质-蛋白质相互作用 (PPI)。
方法
我们获得了包含 HPr 的前 48 个残基的片段 (HPr48)。荧光、远紫外 (UV) 圆二色性 (CD)、小角 X 射线散射 (SAXS) 和核磁共振 (NMR) 显示 HPr48 无序。
结果
来自指定主链核的二级结构倾向进一步支持片段的展开性质。然而,HPr48 能够结合: (i) EI、EIN 的 N 末端区域; (ii) 完整的 Rsd; (iii) HPrK/P,如荧光、远紫外 CD、NMR 和生物层干涉测量法 (BLI) 所示。通过荧光和 BLI 测量的每种蛋白质的关联常数均处于低微摩尔范围内,类似于完整 HPr 和每个其他大分子之间测量的关联常数。
结论
HPr48虽然有48个残基长,但它辅助抗生素发挥抗菌活性。
一般意义
HPr48 可用作开发新抗生素的先导化合物,或者提高现有抗生素的效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号