当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antimicrobial peptides: mechanism of action and lipid-mediated synergistic interactions within membranes
Faraday Discussions ( IF 3.3 ) Pub Date : 2021-09-17 , DOI: 10.1039/d0fd00041h Dennis W Juhl 1 , Elise Glattard 1 , Christopher Aisenbrey 1 , Burkhard Bechinger 1, 2
Faraday Discussions ( IF 3.3 ) Pub Date : 2021-09-17 , DOI: 10.1039/d0fd00041h Dennis W Juhl 1 , Elise Glattard 1 , Christopher Aisenbrey 1 , Burkhard Bechinger 1, 2
Affiliation
|
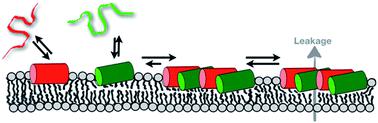
Biophysical and structural studies of peptide–lipid interactions, peptide topology and dynamics have changed our view of how antimicrobial peptides insert and interact with membranes. Clearly, both peptides and lipids are highly dynamic, and change and mutually adapt their conformation, membrane penetration and detailed morphology on a local and a global level. As a consequence, peptides and lipids can form a wide variety of supramolecular assemblies in which the more hydrophobic sequences preferentially, but not exclusively, adopt transmembrane alignments and have the potential to form oligomeric structures similar to those suggested by the transmembrane helical bundle model. In contrast, charged amphipathic sequences tend to stay intercalated at the membrane interface. Although the membranes are soft and can adapt, at increasing peptide density they cause pronounced disruptions of the phospholipid fatty acyl packing. At even higher local or global concentrations the peptides cause transient membrane openings, rupture and ultimately lysis. Interestingly, mixtures of peptides such as magainin 2 and PGLa, which are stored and secreted naturally as a cocktail, exhibit considerably enhanced antimicrobial activities when investigated together in antimicrobial assays and also in pore forming experiments applied to biophysical model systems. Our most recent investigations reveal that these peptides do not form stable complexes but act by specific lipid-mediated interactions and the nanoscale properties of phospholipid bilayers.
中文翻译:

抗菌肽:作用机制和脂质介导的膜内协同作用
肽-脂质相互作用、肽拓扑结构和动力学的生物物理和结构研究改变了我们对抗菌肽如何插入膜并与膜相互作用的看法。显然,肽和脂质都是高度动态的,并且在局部和全局水平上改变和相互适应它们的构象、膜渗透和详细形态。因此,肽和脂质可以形成各种各样的超分子组装体,其中更疏水的序列优先但不排他地采用跨膜排列,并有可能形成类似于跨膜螺旋束模型所建议的寡聚结构。相反,带电的两亲序列倾向于保持插入膜界面。虽然膜很软,可以适应,在增加肽密度时,它们会导致磷脂脂肪酰基包装的明显破坏。在更高的局部或整体浓度下,肽会导致短暂的膜开口、破裂并最终溶解。有趣的是,像magainin 2 和 PGLa 这样的肽混合物,它们作为混合物自然储存和分泌,在抗菌测定和应用于生物物理模型系统的成孔实验中一起研究时,表现出显着增强的抗菌活性。我们最近的研究表明,这些肽不会形成稳定的复合物,而是通过特定的脂质介导的相互作用和磷脂双层的纳米级特性起作用。在更高的局部或整体浓度下,肽会导致短暂的膜开口、破裂并最终溶解。有趣的是,像magainin 2 和 PGLa 这样的肽混合物,它们作为混合物自然储存和分泌,在抗菌测定和应用于生物物理模型系统的成孔实验中一起研究时,表现出显着增强的抗菌活性。我们最近的研究表明,这些肽不会形成稳定的复合物,而是通过特定的脂质介导的相互作用和磷脂双层的纳米级特性起作用。在更高的局部或整体浓度下,肽会导致短暂的膜开口、破裂并最终溶解。有趣的是,像magainin 2 和 PGLa 这样的肽混合物,它们作为混合物自然储存和分泌,在抗菌测定和应用于生物物理模型系统的成孔实验中一起研究时,表现出显着增强的抗菌活性。我们最近的研究表明,这些肽不会形成稳定的复合物,而是通过特定的脂质介导的相互作用和磷脂双层的纳米级特性起作用。当在抗菌测定中以及在应用于生物物理模型系统的成孔实验中一起研究时,表现出显着增强的抗菌活性。我们最近的研究表明,这些肽不会形成稳定的复合物,而是通过特定的脂质介导的相互作用和磷脂双层的纳米级特性起作用。当在抗菌测定中以及在应用于生物物理模型系统的成孔实验中一起研究时,表现出显着增强的抗菌活性。我们最近的研究表明,这些肽不会形成稳定的复合物,而是通过特定的脂质介导的相互作用和磷脂双层的纳米级特性起作用。
更新日期:2021-09-17
中文翻译:

抗菌肽:作用机制和脂质介导的膜内协同作用
肽-脂质相互作用、肽拓扑结构和动力学的生物物理和结构研究改变了我们对抗菌肽如何插入膜并与膜相互作用的看法。显然,肽和脂质都是高度动态的,并且在局部和全局水平上改变和相互适应它们的构象、膜渗透和详细形态。因此,肽和脂质可以形成各种各样的超分子组装体,其中更疏水的序列优先但不排他地采用跨膜排列,并有可能形成类似于跨膜螺旋束模型所建议的寡聚结构。相反,带电的两亲序列倾向于保持插入膜界面。虽然膜很软,可以适应,在增加肽密度时,它们会导致磷脂脂肪酰基包装的明显破坏。在更高的局部或整体浓度下,肽会导致短暂的膜开口、破裂并最终溶解。有趣的是,像magainin 2 和 PGLa 这样的肽混合物,它们作为混合物自然储存和分泌,在抗菌测定和应用于生物物理模型系统的成孔实验中一起研究时,表现出显着增强的抗菌活性。我们最近的研究表明,这些肽不会形成稳定的复合物,而是通过特定的脂质介导的相互作用和磷脂双层的纳米级特性起作用。在更高的局部或整体浓度下,肽会导致短暂的膜开口、破裂并最终溶解。有趣的是,像magainin 2 和 PGLa 这样的肽混合物,它们作为混合物自然储存和分泌,在抗菌测定和应用于生物物理模型系统的成孔实验中一起研究时,表现出显着增强的抗菌活性。我们最近的研究表明,这些肽不会形成稳定的复合物,而是通过特定的脂质介导的相互作用和磷脂双层的纳米级特性起作用。在更高的局部或整体浓度下,肽会导致短暂的膜开口、破裂并最终溶解。有趣的是,像magainin 2 和 PGLa 这样的肽混合物,它们作为混合物自然储存和分泌,在抗菌测定和应用于生物物理模型系统的成孔实验中一起研究时,表现出显着增强的抗菌活性。我们最近的研究表明,这些肽不会形成稳定的复合物,而是通过特定的脂质介导的相互作用和磷脂双层的纳米级特性起作用。当在抗菌测定中以及在应用于生物物理模型系统的成孔实验中一起研究时,表现出显着增强的抗菌活性。我们最近的研究表明,这些肽不会形成稳定的复合物,而是通过特定的脂质介导的相互作用和磷脂双层的纳米级特性起作用。当在抗菌测定中以及在应用于生物物理模型系统的成孔实验中一起研究时,表现出显着增强的抗菌活性。我们最近的研究表明,这些肽不会形成稳定的复合物,而是通过特定的脂质介导的相互作用和磷脂双层的纳米级特性起作用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号