当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Actinide arene-metalates: ion pairing effects on the electronic structure of unsupported uranium–arenide sandwich complexes
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1sc03275e Jesse Murillo 1 , Rina Bhowmick 2 , Katie L M Harriman 3 , Alejandra Gomez-Torres 1 , Joshua Wright 4 , Robert W Meulenberg 5 , Pere Miró 2 , Alejandro Metta-Magaña 1 , Muralee Murugesu 3 , Bess Vlaisavljevich 2 , Skye Fortier 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1sc03275e Jesse Murillo 1 , Rina Bhowmick 2 , Katie L M Harriman 3 , Alejandra Gomez-Torres 1 , Joshua Wright 4 , Robert W Meulenberg 5 , Pere Miró 2 , Alejandro Metta-Magaña 1 , Muralee Murugesu 3 , Bess Vlaisavljevich 2 , Skye Fortier 1
Affiliation
|
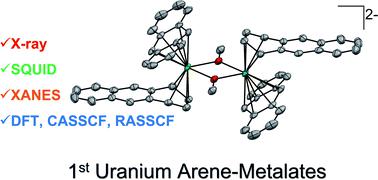
Addition of [UI2(THF)3(μ-OMe)]2·THF (2·THF) to THF solutions containing 6 equiv. of K[C14H10] generates the heteroleptic dimeric complexes [K(18-crown-6)(THF)2]2[U(η6-C14H10)(η4-C14H10)(μ-OMe)]2·4THF (118C6·4THF) and {[K(THF)3][U(η6-C14H10)(η4-C14H10)(μ-OMe)]}2 (1THF) upon crystallization of the products in THF in the presence or absence of 18-crown-6, respectively. Both 118C6·4THF and 1THF are thermally stable in the solid-state at room temperature; however, after crystallization, they become insoluble in THF or DME solutions and instead gradually decompose upon standing. X-ray diffraction analysis reveals 118C6·4THF and 1THF to be structurally similar, possessing uranium centres sandwiched between bent anthracenide ligands of mixed tetrahapto and hexahapto ligation modes. Yet, the two complexes are distinguished by the close contact potassium-arenide ion pairing that is seen in 1THF but absent in 118C6·4THF, which is observed to have a significant effect on the electronic characteristics of the two complexes. Structural analysis, SQUID magnetometry data, XANES spectral characterization, and computational analyses are generally consistent with U(IV) formal assignments for the metal centres in both 118C6·4THF and 1THF, though noticeable differences are detected between the two species. For instance, the effective magnetic moment of 1THF (3.74 μB) is significantly lower than that of 118C6·4THF (4.40 μB) at 300 K. Furthermore, the XANES data shows the U LIII-edge absorption energy for 1THF to be 0.9 eV higher than that of 118C6·4THF, suggestive of more oxidized metal centres in the former. Of note, CASSCF calculations on the model complex {[U(η6-C14H10)(η4-C14H10)(μ-OMe)]2}2− (1*) shows highly polarized uranium–arenide interactions defined by π-type bonds where the metal contributions are primarily comprised by the 6d-orbitals (7.3 ± 0.6%) with minor participation from the 5f-orbitals (1.5 ± 0.5%). These unique complexes provide new insights into actinide–arenide bonding interactions and show the sensitivity of the electronic structures of the uranium atoms to coordination sphere effects.
中文翻译:

锕系芳烃-金属酸盐:离子对对无负载铀-芳烃夹心配合物电子结构的影响
将 [UI 2 (THF) 3 (μ-OMe)] 2 ·THF ( 2 ·THF) 添加到含有 6 当量的 THF 溶液中。K[C 14 H 10 ] 生成杂配二聚体复合物 [K(18-crown-6)(THF) 2 ] 2 [U(η 6 -C 14 H 10 )(η 4 -C 14 H 10 )(μ -OMe)] 2 ·4THF ( 1 18C6 ·4THF) 和 {[K(THF) 3 ][U(η 6 -C 14 H 10 )(η 4 -C 14 H10 )(μ-OMe)]} 2 ( 1 THF ) 分别在存在或不存在 18-crown-6 的情况下在 THF 中结晶产物。两个1 18C6 ·4THF和1 THF是在固体状态在室温下是热稳定的; 然而,结晶后,它们变得不溶于 THF 或 DME 溶液,而是在静置时逐渐分解。X射线衍射分析显示1 18C6 ·4THF和1 THF结构相似,铀中心夹在混合四联体和六联体连接模式的弯曲蒽配体之间。然而,这两种配合物的特点是紧密接触的芳烃钾离子对出现在1 THF 中,但在1 18C6 ·4THF 中不存在,据观察,这对两种配合物的电子特性有显着影响。结构分析、SQUID 磁力测量数据、XANES 光谱表征和计算分析通常与1 18C6 ·4THF 和1 THF 中金属中心的U( IV ) 形式分配一致,尽管在两个物种之间检测到了明显的差异。例如,1 THF (3.74 μ B )的有效磁矩在 300 K 时明显低于1 18C6 ·4THF (4.40 μ B )。此外,XANES 数据显示了1 THF的 UL III边缘吸收能量比1 18C6 ·4THF高 0.9 eV ,表明前者有更多的氧化金属中心。值得注意的是,CASSCF 在模型复合体 {[U(η 6 -C 14 H 10 )(η 4 -C 14 H10 )(μ-OMe)] 2 } 2− ( 1* ) 显示由 π 型键定义的高度极化的铀-芳烃相互作用,其中金属贡献主要由 6d 轨道 (7.3 ± 0.6%) 组成,少量参与来自 5f 轨道 (1.5 ± 0.5%)。这些独特的配合物提供了对锕系-芳烃键相互作用的新见解,并显示了铀原子的电子结构对配位球效应的敏感性。
更新日期:2021-09-17
中文翻译:

锕系芳烃-金属酸盐:离子对对无负载铀-芳烃夹心配合物电子结构的影响
将 [UI 2 (THF) 3 (μ-OMe)] 2 ·THF ( 2 ·THF) 添加到含有 6 当量的 THF 溶液中。K[C 14 H 10 ] 生成杂配二聚体复合物 [K(18-crown-6)(THF) 2 ] 2 [U(η 6 -C 14 H 10 )(η 4 -C 14 H 10 )(μ -OMe)] 2 ·4THF ( 1 18C6 ·4THF) 和 {[K(THF) 3 ][U(η 6 -C 14 H 10 )(η 4 -C 14 H10 )(μ-OMe)]} 2 ( 1 THF ) 分别在存在或不存在 18-crown-6 的情况下在 THF 中结晶产物。两个1 18C6 ·4THF和1 THF是在固体状态在室温下是热稳定的; 然而,结晶后,它们变得不溶于 THF 或 DME 溶液,而是在静置时逐渐分解。X射线衍射分析显示1 18C6 ·4THF和1 THF结构相似,铀中心夹在混合四联体和六联体连接模式的弯曲蒽配体之间。然而,这两种配合物的特点是紧密接触的芳烃钾离子对出现在1 THF 中,但在1 18C6 ·4THF 中不存在,据观察,这对两种配合物的电子特性有显着影响。结构分析、SQUID 磁力测量数据、XANES 光谱表征和计算分析通常与1 18C6 ·4THF 和1 THF 中金属中心的U( IV ) 形式分配一致,尽管在两个物种之间检测到了明显的差异。例如,1 THF (3.74 μ B )的有效磁矩在 300 K 时明显低于1 18C6 ·4THF (4.40 μ B )。此外,XANES 数据显示了1 THF的 UL III边缘吸收能量比1 18C6 ·4THF高 0.9 eV ,表明前者有更多的氧化金属中心。值得注意的是,CASSCF 在模型复合体 {[U(η 6 -C 14 H 10 )(η 4 -C 14 H10 )(μ-OMe)] 2 } 2− ( 1* ) 显示由 π 型键定义的高度极化的铀-芳烃相互作用,其中金属贡献主要由 6d 轨道 (7.3 ± 0.6%) 组成,少量参与来自 5f 轨道 (1.5 ± 0.5%)。这些独特的配合物提供了对锕系-芳烃键相互作用的新见解,并显示了铀原子的电子结构对配位球效应的敏感性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号