当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complexation and redox chemistry of neptunium, plutonium and americium with a hydroxylaminato ligand
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-03 , DOI: 10.1039/d1sc03905a Jing Su 1 , Thibault Cheisson 2 , Alex McSkimming 2 , Conrad A P Goodwin 3 , Ida M DiMucci 3 , Thomas Albrecht-Schönzart 4 , Brian L Scott 5 , Enrique R Batista 1 , Andrew J Gaunt 3 , Stosh A Kozimor 3 , Ping Yang 1 , Eric J Schelter 2
Chemical Science ( IF 7.6 ) Pub Date : 2021-09-03 , DOI: 10.1039/d1sc03905a Jing Su 1 , Thibault Cheisson 2 , Alex McSkimming 2 , Conrad A P Goodwin 3 , Ida M DiMucci 3 , Thomas Albrecht-Schönzart 4 , Brian L Scott 5 , Enrique R Batista 1 , Andrew J Gaunt 3 , Stosh A Kozimor 3 , Ping Yang 1 , Eric J Schelter 2
Affiliation
|
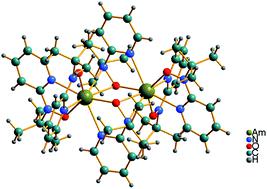
There is significant interest in ligands that can stabilize actinide ions in oxidation states that can be exploited to chemically differentiate 5f and 4f elements. Applications range from developing large-scale actinide separation strategies for nuclear industry processing to carrying out analytical studies that support environmental monitoring and remediation efforts. Here, we report syntheses and characterization of Np(IV), Pu(IV) and Am(III) complexes with N-tert-butyl-N-(pyridin-2-yl)hydroxylaminato, [2-(tBuNO)py]−(interchangeable hereafter with [(tBuNO)py]−), a ligand which was previously found to impart remarkable stability to cerium in the +4 oxidation state. An[(tBuNO)py]4 (An = Pu, 1; Np, 2) have been synthesized, characterized by X-ray diffraction, X-ray absorption, 1H NMR and UV-vis-NIR spectroscopies, and cyclic voltammetry, along with computational modeling and analysis. In the case of Pu, oxidation of Pu(III) to Pu(IV) was observed upon complexation with the [(tBuNO)py]− ligand. The Pu complex 1 and Np complex 2 were also isolated directly from Pu(IV) and Np(IV) precursors. Electrochemical measurements indicate that a Pu(III) species can be accessed upon one-electron reduction of 1 with a large negative reduction potential (E1/2 = −2.26 V vs. Fc+/0). Applying oxidation potentials to 1 and 2 resulted in ligand-centered electron transfer reactions, which is different from the previously reported redox chemistry of UIV[(tBuNO)py]4 that revealed a stable U(V) product. Treatment of an anhydrous Am(III) precursor with the [(tBuNO)py]− ligand did not result in oxidation to Am(IV). Instead, the dimeric complex [AmIII(μ2-(tBuNO)py)((tBuNO)py)2]2 (3) was isolated. Complex 3 is a rare example of a structurally characterized non-aqueous Am-containing molecular complex prepared using inert atmosphere techniques. Predicted redox potentials from density functional theory calculations show a trivalent accessibility trend of U(III) < Np(III) < Pu(III) and that the higher oxidation states of actinides (i.e., +5 for Np and Pu and +4 for Am) are not stabilized by [2-(tBuNO)py]−, in good agreement with experimental observations.
中文翻译:

镎、钚和镅与羟基层合配体的络合和氧化还原化学
人们对可以稳定氧化态的锕系元素离子的配体非常感兴趣,这些配体可用于化学区分 5f 和 4f 元素。应用范围从开发用于核工业加工的大规模锕系元素分离策略到进行支持环境监测和修复工作的分析研究。这里,我们报告合成和Np个表征(IV),普(IV)和AM(III)配合物的N-叔丁基- ñ - (吡啶-2-基)hydroxylaminato,[2-(吨BUNO)吡啶] -(以后可与 [( t BuNO)py互换] -),一种先前被发现赋予+4氧化态铈显着稳定性的配体。An[( t BuNO)py] 4 (An = Pu, 1 ; Np, 2 ) 已合成,通过 X 射线衍射、X 射线吸收、1 H NMR 和 UV-vis-NIR 光谱以及循环伏安法表征,以及计算建模和分析。在Pu的情况下,在与[( t BuNO)py] -配体络合后观察到Pu( III )氧化成Pu( IV ) 。Pu复合物1和Np复合物2也直接从Pu( IV )和Np(IV ) 前体。电化学测量表明可以通过单电子还原1获得 Pu( III ) 物质,并具有大的负还原电位(E 1/2 = -2.26 V vs. Fc +/0)。将氧化电位应用于1和2导致以配体为中心的电子转移反应,这与之前报道的 U IV [( t BuNO)py] 4 的氧化还原化学不同,后者显示出稳定的 U( V ) 产物。用 [( t BuNO)py]处理无水 Am( III ) 前体-配体不会导致氧化为 Am( IV )。相反,二聚体复合物[Am III (μ 2 -( t BuNO)py)(( t BuNO)py) 2 ] 2 ( 3 )被分离。配合物3是使用惰性气氛技术制备的结构表征的非水性含 Am 分子配合物的罕见例子。来自密度泛函理论计算的预测氧化还原电位显示出 U( III ) < Np( III ) < Pu( III )的三价可及性趋势,并且锕系元素的较高氧化态(即, Np 和 Pu 的 +5 和 Am 的 +4) 不受 [2-( t BuNO)py] - 的稳定,与实验观察结果非常一致。
更新日期:2021-09-17
中文翻译:

镎、钚和镅与羟基层合配体的络合和氧化还原化学
人们对可以稳定氧化态的锕系元素离子的配体非常感兴趣,这些配体可用于化学区分 5f 和 4f 元素。应用范围从开发用于核工业加工的大规模锕系元素分离策略到进行支持环境监测和修复工作的分析研究。这里,我们报告合成和Np个表征(IV),普(IV)和AM(III)配合物的N-叔丁基- ñ - (吡啶-2-基)hydroxylaminato,[2-(吨BUNO)吡啶] -(以后可与 [( t BuNO)py互换] -),一种先前被发现赋予+4氧化态铈显着稳定性的配体。An[( t BuNO)py] 4 (An = Pu, 1 ; Np, 2 ) 已合成,通过 X 射线衍射、X 射线吸收、1 H NMR 和 UV-vis-NIR 光谱以及循环伏安法表征,以及计算建模和分析。在Pu的情况下,在与[( t BuNO)py] -配体络合后观察到Pu( III )氧化成Pu( IV ) 。Pu复合物1和Np复合物2也直接从Pu( IV )和Np(IV ) 前体。电化学测量表明可以通过单电子还原1获得 Pu( III ) 物质,并具有大的负还原电位(E 1/2 = -2.26 V vs. Fc +/0)。将氧化电位应用于1和2导致以配体为中心的电子转移反应,这与之前报道的 U IV [( t BuNO)py] 4 的氧化还原化学不同,后者显示出稳定的 U( V ) 产物。用 [( t BuNO)py]处理无水 Am( III ) 前体-配体不会导致氧化为 Am( IV )。相反,二聚体复合物[Am III (μ 2 -( t BuNO)py)(( t BuNO)py) 2 ] 2 ( 3 )被分离。配合物3是使用惰性气氛技术制备的结构表征的非水性含 Am 分子配合物的罕见例子。来自密度泛函理论计算的预测氧化还原电位显示出 U( III ) < Np( III ) < Pu( III )的三价可及性趋势,并且锕系元素的较高氧化态(即, Np 和 Pu 的 +5 和 Am 的 +4) 不受 [2-( t BuNO)py] - 的稳定,与实验观察结果非常一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号