当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
General Efficacy of Atomically Dispersed Pt Catalysts for the Chlorine Evolution Reaction: Potential-Dependent Switching of the Kinetics and Mechanism
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-17 , DOI: 10.1021/acscatal.1c03893 Taejung Lim 1 , Jae Hyung Kim 1 , Jinjong Kim 2 , Du San Baek 1 , Tae Joo Shin 3 , Hu Young Jeong 3 , Kug-Seung Lee 4 , Kai S. Exner 5, 6, 7 , Sang Hoon Joo 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-17 , DOI: 10.1021/acscatal.1c03893 Taejung Lim 1 , Jae Hyung Kim 1 , Jinjong Kim 2 , Du San Baek 1 , Tae Joo Shin 3 , Hu Young Jeong 3 , Kug-Seung Lee 4 , Kai S. Exner 5, 6, 7 , Sang Hoon Joo 2
Affiliation
|
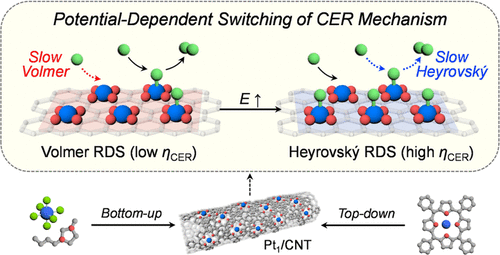
The electrochemical chlorine evolution reaction (CER) is a key anodic reaction in the chlor-alkali process for Cl2 production, on-site generation of ClO–, and Cl2-mediated electrosynthesis. Although Ru-based mixed metal oxides have long been used as CER catalysts, they suffer from a selectivity problem due to the competing oxygen evolution reaction. To overcome this shortcoming, we have developed a new CER catalyst composed of atomically dispersed Pt–N4 sites on carbon nanotubes (Pt1/CNT). In this study, we demonstrate that the catalytically active Pt–N4 sites can be constructed from H2PtCl6·6H2O and an ionic liquid via a bottom-up approach and a Pt-porphyrin-driven top-down method. Both catalysts exhibit excellent CER activity and remarkable selectivity, demonstrating the general efficacy of Pt1/CNT for the CER. The electrochemical and in situ X-ray absorption spectroscopy analyses reveal that Pt1/CNT catalysts show a reaction order of ∼1.8 in the low overpotential regime, where the Volmer step is reconciled with the rate-determining step (RDS). Interestingly, in the high overpotential region, the CER over Pt1/CNT proceeds with a lower reaction order and the RDS switches to the Heyrovský step. These unprecedented kinetic insights are clearly distinguished from the oxide-based CER catalysts with the opposite sequence of the RDS.
中文翻译:

用于氯演化反应的原子分散 Pt 催化剂的一般功效:动力学和机制的电位相关转换
电化学析氯反应 (CER) 是氯碱工艺中用于生产Cl 2、现场生成 ClO –和 Cl 2介导的电合成的关键阳极反应。尽管基于 Ru 的混合金属氧化物长期以来一直被用作 CER 催化剂,但由于竞争性析氧反应,它们存在选择性问题。为了克服这个缺点,我们开发了一种新的 CER 催化剂,它由碳纳米管上原子分散的 Pt-N 4位点(Pt 1 /CNT)组成。在这项研究中,我们证明了催化活性 Pt-N 4位点可以由 H 2 PtCl 6 ·6H 2构建O 和离子液体通过自下而上的方法和 Pt-卟啉驱动的自上而下的方法。两种催化剂都表现出优异的 CER 活性和显着的选择性,证明了 Pt 1 /CNT 对 CER的一般功效。电化学和原位 X 射线吸收光谱分析表明,Pt 1 /CNT 催化剂在低过电位状态下显示出约 1.8 的反应级数,其中 Volmer 步骤与速率决定步骤(RDS)相协调。有趣的是,在高过电位区域,Pt 1 /CNT 上的 CER以较低的反应顺序进行,RDS 切换到 Heyrovský 步骤。这些前所未有的动力学见解与具有相反顺序的 RDS 的氧化物基 CER 催化剂明显不同。
更新日期:2021-10-01
中文翻译:

用于氯演化反应的原子分散 Pt 催化剂的一般功效:动力学和机制的电位相关转换
电化学析氯反应 (CER) 是氯碱工艺中用于生产Cl 2、现场生成 ClO –和 Cl 2介导的电合成的关键阳极反应。尽管基于 Ru 的混合金属氧化物长期以来一直被用作 CER 催化剂,但由于竞争性析氧反应,它们存在选择性问题。为了克服这个缺点,我们开发了一种新的 CER 催化剂,它由碳纳米管上原子分散的 Pt-N 4位点(Pt 1 /CNT)组成。在这项研究中,我们证明了催化活性 Pt-N 4位点可以由 H 2 PtCl 6 ·6H 2构建O 和离子液体通过自下而上的方法和 Pt-卟啉驱动的自上而下的方法。两种催化剂都表现出优异的 CER 活性和显着的选择性,证明了 Pt 1 /CNT 对 CER的一般功效。电化学和原位 X 射线吸收光谱分析表明,Pt 1 /CNT 催化剂在低过电位状态下显示出约 1.8 的反应级数,其中 Volmer 步骤与速率决定步骤(RDS)相协调。有趣的是,在高过电位区域,Pt 1 /CNT 上的 CER以较低的反应顺序进行,RDS 切换到 Heyrovský 步骤。这些前所未有的动力学见解与具有相反顺序的 RDS 的氧化物基 CER 催化剂明显不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号