当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Lewis Acid-Catalyzed Diastereoselective Synthesis of Functionalized 2-Diazo-1,5-dicarbonyl Compounds
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-16 , DOI: 10.1021/acscatal.1c03036 Evan M. Howard 1 , Matthias Brewer 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-09-16 , DOI: 10.1021/acscatal.1c03036 Evan M. Howard 1 , Matthias Brewer 1
Affiliation
|
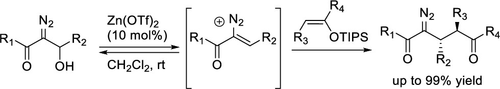
A diverse array of 2-diazo-1,5-dicarbonyl compounds were formed by the Lewis acid-catalyzed reaction of enoxysilanes with β-hydroxy-α-diazo carbonyls. This reaction proceeds via the Zn(OTf)2-catalyzed dehydroxylation of the β-hydroxy-α-diazo carbonyl to form a vinyl diazonium ion intermediate that is intercepted by the enoxysilane nucleophile to give diazo-containing scaffolds with increased molecular complexity. The reaction appears to be general, and a variety of functional groups, including common protecting groups, are well tolerated. The 2-diazo-1,5-dicarbonyl products are formed in yields of up to 99% with diastereoselectivity up to >20:1.
中文翻译:

路易斯酸催化的功能化 2-重氮-1,5-二羰基化合物的非对映选择性合成
通过路易斯酸催化的烯氧基硅烷与 β-羟基-α-重氮羰基化合物的反应,形成了多种 2-重氮-1,5-二羰基化合物。该反应通过 Zn(OTf) 2催化的 β-羟基-α-重氮羰基脱羟基作用进行,形成乙烯基重氮离子中间体,该中间体被烯氧基硅烷亲核试剂截获,得到分子复杂性增加的含重氮支架。该反应似乎很普遍,并且可以很好地耐受各种官能团,包括常见的保护基团。2-重氮-1,5-二羰基产物的产率高达 99%,非对映选择性高达 >20:1。
更新日期:2021-10-01
中文翻译:

路易斯酸催化的功能化 2-重氮-1,5-二羰基化合物的非对映选择性合成
通过路易斯酸催化的烯氧基硅烷与 β-羟基-α-重氮羰基化合物的反应,形成了多种 2-重氮-1,5-二羰基化合物。该反应通过 Zn(OTf) 2催化的 β-羟基-α-重氮羰基脱羟基作用进行,形成乙烯基重氮离子中间体,该中间体被烯氧基硅烷亲核试剂截获,得到分子复杂性增加的含重氮支架。该反应似乎很普遍,并且可以很好地耐受各种官能团,包括常见的保护基团。2-重氮-1,5-二羰基产物的产率高达 99%,非对映选择性高达 >20:1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号