Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2021-09-17 , DOI: 10.1016/j.jcis.2021.09.076 Miftah Hidayat 1 , Mohammad Sarmadivaleh 2 , Jos Derksen 3 , David Vega-Maza 4 , Stefan Iglauer 5 , Jan Vinogradov 3
|
Despite the broad range of interest and applications, controls on the electric surface charge and the zeta potential of silica in contact with aqueous solutions saturated with dissolved CO2 at conditions relevant to natural systems, remains unreported. There have been no published zeta potential measurements conducted in such systems at equilibrium, hence the effect of composition, pH, temperature and pressure remains unknown.
We describe a novel methodology developed for the streaming potential measurements under these conditions, and report zeta potential values for the first time obtained with Fontainebleau sandstone core sample saturated with carbonated NaCl, Na2SO4, CaCl2 and MgCl2 solutions under equilibrium conditions at pressures up to 10 MPa and temperatures up to 40 °C.
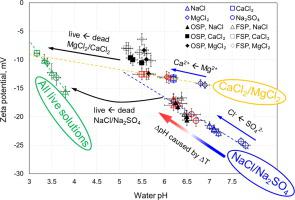
The results demonstrate that pH of solutions is the only control on the zeta potential, while temperature, CO2 pressure and salt type affect pH values. We report three empirical relationships that describe the pH dependence of the zeta potential for: i) dead (partial CO2 pressure of 10-3.44 atm) NaCl/Na2SO4, ii) dead CaCl2/MgCl2 solutions, and iii) for all live (fully saturated with dissolved CO2) solutions. The proposed new relationships provide essential insights into interfacial electrochemical properties of silica-water systems at conditions relevant to CO2 geological storage.
中文翻译:

在 23 °C 和 40 °C 的温度和高达 10.0 MPa 的压力下与完整砂岩样品接触的富含 CO2 的水溶液的 Zeta 电位
尽管具有广泛的兴趣和应用,但在与自然系统相关的条件下,对与溶解的 CO 2饱和的水溶液接触的二氧化硅的表面电荷和 zeta 电位的控制仍然没有报道。尚未公开在此类系统中进行平衡时的 zeta 电位测量,因此成分、pH、温度和压力的影响仍然未知。
我们描述了在这些条件下为流电势测量开发的新方法,并报告了第一次用枫丹白露砂岩岩心样品在平衡条件下用碳酸化 NaCl、Na 2 SO 4、CaCl 2和 MgCl 2溶液在压力高达 10 MPa,温度高达 40 °C。
结果表明,溶液的 pH 值是对 zeta 电位的唯一控制,而温度、CO 2压力和盐类型会影响 pH 值。我们报告了三个经验关系,这些关系描述了 zeta 电位的 pH 依赖性:i) 死的(部分 CO 2压力为 10 -3.44 atm)NaCl/Na 2 SO 4,ii) 死的 CaCl 2 /MgCl 2溶液,以及 iii)适用于所有活性(溶解的 CO 2完全饱和)溶液。拟议的新关系为二氧化硅-水系统在与 CO 2地质储存相关的条件下的界面电化学特性提供了重要的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号