当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Advantages of pH and Temperature Control in the Carbonation Stage for Li2CO3 Production with Sulphated Liquors
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-09-17 , DOI: 10.1002/slct.202101873 Daniel Mendieta‐George 1 , Roberto Pérez‐Garibay 1 , Ricardo Solís‐Rodríguez 1 , Juan C. Rendón‐Ángeles 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2021-09-17 , DOI: 10.1002/slct.202101873 Daniel Mendieta‐George 1 , Roberto Pérez‐Garibay 1 , Ricardo Solís‐Rodríguez 1 , Juan C. Rendón‐Ángeles 1
Affiliation
|
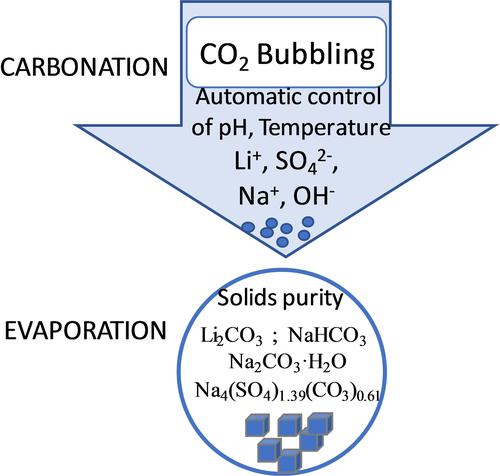
This work addresses the carbonation of an aqueous solution that simulates leach liquor with sulphuric acid, a chemical system that is seldom studied, and the subsequent precipitation of Li2CO3 via evaporation of the diluent water. New details were revealed on the automatic control of pH and temperature to optimizes the carbonation operation conditions and the quality of the products. Among the relevant findings are: a) the carbonation stage was optimized at pH 12 and 35 °C because, under these conditions, the redox potential (100–200 mV) promotes the carbonates stability, b) the carbonation reaction in sulphated solutions has zero order and its activation energy is 6.81 kJ/mol, suggesting that the reaction is controlled by diffusion, and c) At pH 8 and 20 °C the maximum purity (99.9 % Li2CO3) was precipitated. Additionally, other carbonate based secondary phases were precipitated together with Li2CO3. The chemical stability of these secondary phases is pH dependent and are discussed in detail.
中文翻译:

硫酸盐生产 Li2CO3 碳酸化阶段 pH 和温度控制的优势
这项工作解决了用硫酸模拟浸出液的水溶液的碳酸化,这是一种很少研究的化学系统,以及随后的 Li 2 CO 3沉淀通过稀释水的蒸发。揭示了pH和温度自动控制以优化碳酸化操作条件和产品质量的新细节。相关发现包括:a) 碳酸化阶段在 pH 12 和 35°C 下进行了优化,因为在这些条件下,氧化还原电位 (100-200 mV) 促进了碳酸盐的稳定性,b) 硫酸盐溶液中的碳酸化反应为零顺序,其活化能为 6.81 kJ/mol,表明反应受扩散控制,并且 c) 在 pH 8 和 20 °C 下,最大纯度 (99.9 % Li 2 CO 3 ) 沉淀。此外,其他基于碳酸盐的第二相与 Li 2 CO 3一起沉淀. 这些第二相的化学稳定性取决于 pH 值,并进行了详细讨论。
更新日期:2021-09-17
中文翻译:

硫酸盐生产 Li2CO3 碳酸化阶段 pH 和温度控制的优势
这项工作解决了用硫酸模拟浸出液的水溶液的碳酸化,这是一种很少研究的化学系统,以及随后的 Li 2 CO 3沉淀通过稀释水的蒸发。揭示了pH和温度自动控制以优化碳酸化操作条件和产品质量的新细节。相关发现包括:a) 碳酸化阶段在 pH 12 和 35°C 下进行了优化,因为在这些条件下,氧化还原电位 (100-200 mV) 促进了碳酸盐的稳定性,b) 硫酸盐溶液中的碳酸化反应为零顺序,其活化能为 6.81 kJ/mol,表明反应受扩散控制,并且 c) 在 pH 8 和 20 °C 下,最大纯度 (99.9 % Li 2 CO 3 ) 沉淀。此外,其他基于碳酸盐的第二相与 Li 2 CO 3一起沉淀. 这些第二相的化学稳定性取决于 pH 值,并进行了详细讨论。











































 京公网安备 11010802027423号
京公网安备 11010802027423号