Chinese Journal of Analytical Chemistry ( IF 1.2 ) Pub Date : 2021-09-17 , DOI: 10.1016/j.cjac.2021.07.003 Zai-Ming Qiu 1 , Fei Liu 2 , Zhi-Wu Yu 1 , Xin-Yu Li 1
|
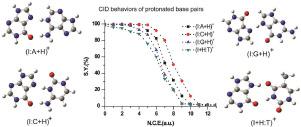
Hypoxanthine (I) is the nucleobase of inosine. In this work, we employed second-order Møller-Plesset perturbation(MP2) method to investigate the protonation properties of hypoxanthine(I), adenine(A), cytosine (C), guanine (G), and thymine (T). The order of proton affinity is as follows: cytosine (N3 and O2(N3) side) ≈ guanine (N7) > adenine (N1) > hypoxanthine (N7) > thymine(O4(N3 side)). To compare the stability of protonated base pairs between I and DNA bases, we investigated the collision-induced dissociation (CID) behavior of the pairs using tandem mass spectrometry method. And the full geometry optimization has been performed for the studied complexes by the MP2 method. Based on the CID results and calculated structures, the stability of the protonated base pairs in DNA decreased in the following order: (I:A+H)+ > (I:C+H)+ > (I:G+H)+ > (I+H:T)+.
中文翻译:

用 MS 和 MP2 方法研究次黄嘌呤和 DNA 碱基之间的质子化碱基对
次黄嘌呤 (I) 是肌苷的核碱基。在这项工作中,我们采用二阶 Møller-Plesset 微扰 (MP2) 方法来研究次黄嘌呤 (I)、腺嘌呤 (A)、胞嘧啶 (C)、鸟嘌呤 (G) 和胸腺嘧啶 (T) 的质子化特性。质子亲和力的顺序如下:胞嘧啶(N 3和O 2(N 3)侧)≈鸟嘌呤(N 7)>腺嘌呤(N 1)>次黄嘌呤(N 7)>胸腺嘧啶(O 4(N 3 )边))。为了比较 I 和 DNA 碱基之间质子化碱基对的稳定性,我们使用串联质谱法研究了这些对的碰撞诱导解离 (CID) 行为。并且通过MP2方法对所研究的配合物进行了全几何优化。根据 CID 结果和计算结构,DNA 中质子化碱基对的稳定性按以下顺序降低:(I:A+H) + > (I:C+H) + > (I:G+H) + > (I+H:T) +。









































 京公网安备 11010802027423号
京公网安备 11010802027423号