Water Research ( IF 11.4 ) Pub Date : 2021-09-17 , DOI: 10.1016/j.watres.2021.117672 Qi Zhao 1 , Wanqian Guo 1 , Haichao Luo 1 , Chuanming Xing 1 , Huazhe Wang 1 , Banghai Liu 1 , Qishi Si 1 , Nanqi Ren 1
|
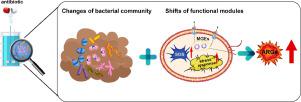
Antibiotics can exert selective pressures on sludge as well as affect the emergence and spread of antibiotic resistance genes (ARGs). However, the underlying mechanisms of ARGs transfers are still controversial and not fully understood in sludge system. In present study, two anaerobic sequence batch reactors (ASBR) were constructed to investigate the development of ARGs exposed to two sulfonamide antibiotics (SMs, sulfadiazine SDZ and sulfamethoxazole SMX) with increasing concentrations. The abundance of corresponding ARGs and total ARGs obviously increased with presence of SMs. Functional analyses indicated that oxidative stress response, signal transduction and type IV secretion systems were triggered by SMs, which would promote ARGs transfers. Network analysis revealed 18 genera were possible hosts of ARGs, and their abundances increased with SMs. Partial least-squares path modeling suggested functional modules directly influenced mobile genetic elements (MGEs) as well as the ARGs might be driven by both functional modules and bacteria community, while bacteria community composition played a more key role. Sludge with refractory antibiotics (SDZ) may stimulate the relevant functions and shift the microbial composition to a greater extent, causing more ARGs to emerge and spread. The mechanisms of ARGs transfers are revealed from the perspective of functional modules and bacterial community in sludge system for the first time, and it could provide beneficial directions, such as oxidative stress reduction, cellular communication control, bacterial composition directional regulation, for ARGs spread controlling in the future.
中文翻译:

解读抗生素暴露条件下抗生素抗性基因的转移:由功能模块和细菌群落驱动
抗生素可以对污泥施加选择压力,并影响抗生素抗性基因 (ARG) 的出现和传播。然而,ARGs 转移的潜在机制仍然存在争议,并且在污泥系统中尚未完全了解。在本研究中,构建了两个厌氧序批式反应器 (ASBR) 来研究 ARGs 暴露于浓度增加的两种磺胺类抗生素(SM、磺胺嘧啶 SDZ 和磺胺甲恶唑 SMX)的发展。相应的 ARG 和总 ARG 的丰度随着 SM 的存在而明显增加。功能分析表明氧化应激反应、信号转导和IV型分泌系统是由SMs触发的,这将促进ARGs的转移。网络分析显示 18 个属可能是 ARGs 的宿主,它们的丰度随着 SM 的增加而增加。偏最小二乘路径建模表明功能模块直接影响移动遗传元件(MGEs)以及 ARGs 可能受功能模块和细菌群落的驱动,而细菌群落组成起着更关键的作用。含有难治性抗生素(SDZ)的污泥可能会刺激相关功能并更大程度地改变微生物组成,导致更多的 ARG 出现和传播。首次从污泥系统功能模块和细菌群落的角度揭示了ARGs转移的机制,可为ARGs的扩散控制提供有益的方向,如氧化应激降低、细胞通讯控制、细菌组成定向调控在将来。偏最小二乘路径建模表明功能模块直接影响移动遗传元件(MGEs)以及 ARGs 可能受功能模块和细菌群落的驱动,而细菌群落组成起着更关键的作用。含有难治性抗生素(SDZ)的污泥可能会刺激相关功能并更大程度地改变微生物组成,导致更多的 ARG 出现和传播。首次从污泥系统功能模块和细菌群落的角度揭示了ARGs转移的机制,可为ARGs的扩散控制提供有益的方向,如氧化应激降低、细胞通讯控制、细菌组成定向调控在将来。偏最小二乘路径建模表明功能模块直接影响移动遗传元件(MGEs)以及 ARGs 可能受功能模块和细菌群落的驱动,而细菌群落组成起着更关键的作用。含有难治性抗生素(SDZ)的污泥可能会刺激相关功能并更大程度地改变微生物组成,导致更多的 ARG 出现和传播。首次从污泥系统功能模块和细菌群落的角度揭示了ARGs转移的机制,可为ARGs的扩散控制提供有益的方向,如氧化应激降低、细胞通讯控制、细菌组成定向调控在将来。







































 京公网安备 11010802027423号
京公网安备 11010802027423号