当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalyst-free and multicomponent synthesis of 3-aminoalkylated indoles via a Mannich-type reaction: multitargeted anticancer, tyrosinase and α-glucosidase inhibitory activities
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-08-26 , DOI: 10.1039/d1nj02536h Thi-Kim-Chi Huynh 1, 2 , Kim-Khanh-Huy Ngo 3 , Hoang-Phuc Nguyen 1, 3 , Hoai-Khanh Dang 3 , Van-Trung Phung 4 , Khac-Minh Thai 5 , Thi-Kim-Dung Hoang 1, 2
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-08-26 , DOI: 10.1039/d1nj02536h Thi-Kim-Chi Huynh 1, 2 , Kim-Khanh-Huy Ngo 3 , Hoang-Phuc Nguyen 1, 3 , Hoai-Khanh Dang 3 , Van-Trung Phung 4 , Khac-Minh Thai 5 , Thi-Kim-Dung Hoang 1, 2
Affiliation
|
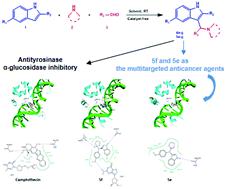
A highly efficient and sustainable approach for the multi-component synthesis of 3-aminoalkylated indoles was investigated via a Mannich-type reaction under catalyst-free conditions, with methanol and ethylene glycol (EG) as the promoting media. Synthesizing various substrates without using column chromatography is time-saving and convenient, which is one of the advantages of this method. In addition, the environmental friendliness is also considered in this research. The synthetic indole derivatives 4a–g and 5a–g, confirmed via spectral analysis, were investigated for their in vitro anticancer activity on human cancer cell lines such as lung (A549), breast (MDA-MB-231) and prostate (PC3) as well as their antityrosinase and α-glucosidase inhibitory activities. Specifically, the results showed that most of the synthesized indoles inhibited both carcinoma and adenocarcinoma cell lines and affected the cytotoxic activity against all three cancer cell lines in the same fashion; moreover, compounds 5e and 5f were shown to be multitargeted anticancer agents. The results of molecular docking studies clarified that the binding of the most potent compounds, 5e and 5f, at the TopI–DNA binding site was through hydrogen bonding as well as hydrophobic interactions and is comparable to camptothecin binding.
中文翻译:

通过曼尼希型反应无催化剂和多组分合成 3-氨基烷基化吲哚:多靶点抗癌、酪氨酸酶和 α-葡萄糖苷酶抑制活性
以甲醇和乙二醇 (EG) 作为促进介质,在无催化剂条件下通过曼尼希型反应研究了一种高效且可持续的 3-氨基烷基化吲哚多组分合成方法。无需柱层析即可合成各种底物,省时、方便,是该方法的优点之一。此外,本研究还考虑了环境友好性。通过光谱分析证实合成的吲哚衍生物4a-g和5a-g进行了体外研究对人癌细胞系如肺癌 (A549)、乳腺癌 (MDA-MB-231) 和前列腺 (PC3) 的抗癌活性以及它们的抗酪氨酸酶和 α-葡萄糖苷酶抑制活性。具体而言,结果表明,大多数合成的吲哚均抑制癌细胞系和腺癌细胞系,并以相同的方式影响对所有三种癌细胞系的细胞毒活性;此外,化合物5e和5f被证明是多靶点抗癌剂。分子对接研究的结果表明,最有效的化合物5e和5f与 TopI-DNA 结合位点的结合是通过氢键和疏水相互作用进行的,与喜树碱结合相当。
更新日期:2021-09-17
中文翻译:

通过曼尼希型反应无催化剂和多组分合成 3-氨基烷基化吲哚:多靶点抗癌、酪氨酸酶和 α-葡萄糖苷酶抑制活性
以甲醇和乙二醇 (EG) 作为促进介质,在无催化剂条件下通过曼尼希型反应研究了一种高效且可持续的 3-氨基烷基化吲哚多组分合成方法。无需柱层析即可合成各种底物,省时、方便,是该方法的优点之一。此外,本研究还考虑了环境友好性。通过光谱分析证实合成的吲哚衍生物4a-g和5a-g进行了体外研究对人癌细胞系如肺癌 (A549)、乳腺癌 (MDA-MB-231) 和前列腺 (PC3) 的抗癌活性以及它们的抗酪氨酸酶和 α-葡萄糖苷酶抑制活性。具体而言,结果表明,大多数合成的吲哚均抑制癌细胞系和腺癌细胞系,并以相同的方式影响对所有三种癌细胞系的细胞毒活性;此外,化合物5e和5f被证明是多靶点抗癌剂。分子对接研究的结果表明,最有效的化合物5e和5f与 TopI-DNA 结合位点的结合是通过氢键和疏水相互作用进行的,与喜树碱结合相当。











































 京公网安备 11010802027423号
京公网安备 11010802027423号