当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and properties of 1,2,3-diazapnictol-5-yl substituted ferrocenes
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-08-25 , DOI: 10.1039/d1nj02666f Pavel Kozáček 1 , Libor Dostál 1 , Martin Hejda 1 , Tomáš Mikysek 2 , Aleš Růžička 1 , Milan Erben 1
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-08-25 , DOI: 10.1039/d1nj02666f Pavel Kozáček 1 , Libor Dostál 1 , Martin Hejda 1 , Tomáš Mikysek 2 , Aleš Růžička 1 , Milan Erben 1
Affiliation
|
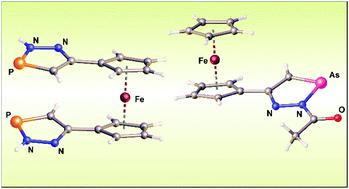
The reaction of acetylferrocene hydrazone or 1,1′-diacetylferrocene dihydrazone with PCl3 gives the corresponding azines as the only organometallic product. The separation of the hydrazone function with a 1,4-phenylene or propane-2,2-diyl spacer enables the preparation of new heterocyclic compounds [(2H-1,2,3-diazaphosphol-5-yl)-4-phenyl]ferrocene (2b) and [1-(2H-1,2,3-diazaphosphol-5-yl)-1-methyl-ethyl]ferrocene (2c) but in low yields. Using acetylated hydrazones, that do not undergo transformation into stable azine molecules, led to the synthesis of (2-acetyl-1,2,3-diazaphosphol-5-yl)ferrocene (5a) and 1,1′-bis(2-acetyl-1,2,3-diazaphosphol-5-yl)ferrocene (8) in up to 70% yield. N-Acylated compounds 5a and 8 are readily converted to (2H-1,2,3-diazaphosphol-5-yl)ferrocene (2a) and 1,1′-bis(2H-1,2,3-diazaphosphol-5-yl)ferrocene (9). Analogous reactions were utilized for the preparation of (2-acetyl-1,2,3-diazarsol-5-yl)ferrocene (10), (2H-1,2,3-diazarsol-5-yl)ferrocene (11), 1,1′-bis(2-acetyl-1,2,3-diazarsol-5-yl)ferrocene (12) and 1,1′-bis(2H-1,2,3-diazarsol-5-yl)ferrocene (13). Electrochemical studies on a series of new ferrocenes showed that the 2H-1,2,3-diazapnictol-5-yl substituent possesses weak electron-withdrawing ability which is enhanced by acetylation in the 2-position of such a heterocycle. We also observed the electroactivity of the 1,2,3-diazapnictolyl substituent as it could be irreversibly reduced at potentials from −1.97 to −2.93 V vs. the Fc0/+ couple. All prepared compounds have been fully characterized using spectroscopic methods and molecular structures of 2a, 2b, 2c, 5a, 8, 9 and 10 are reported.
中文翻译:

1,2,3-二氮杂萘酚-5-基取代二茂铁的合成及性质
乙酰二茂铁腙或1,1'-二乙酰二茂铁二腙与PCl 3 的反应得到相应的吖嗪作为唯一的有机金属产物。腙功能与 1,4-亚苯基或丙烷-2,2-二基间隔基的分离使得能够制备新的杂环化合物 [(2 H -1,2,3-二氮杂磷-5-基)-4-苯基]二茂铁( 2b )和[1-( 2H -1,2,3-二氮杂磷-5-基)-1-甲基-乙基]二茂铁( 2c )但产率低。使用不会转化为稳定的吖嗪分子的乙酰化腙,合成了(2-乙酰-1,2,3-二氮杂磷-5-基)二茂铁(5a)和1,1'-双(2-)乙酰-1,2,3-二氮杂磷-5-基)二茂铁 ( 8) 的产率高达 70%。N-酰化化合物5a和8很容易转化为 (2 H -1,2,3-二氮杂磷-5-基)二茂铁 ( 2a ) 和 1,1'-双 (2 H -1,2,3-二氮杂磷- 5-基)二茂铁( 9 )。类似的反应用于制备 (2-乙酰基-1,2,3-二氮杂-5-基)二茂铁 ( 10 ), ( 2 H -1,2,3-二氮杂- 5-基)二茂铁 ( 11 ) , 1,1'-双(2-乙酰基-1,2,3-二氮杂-5-基)二茂铁( 12 )和1,1'-双(2 H -1,2,3-二氮杂-5-基) )二茂铁 ( 13 )。一系列新二茂铁的电化学研究表明,2H -1,2,3-diazapnictol-5-yl 取代基具有弱的吸电子能力,通过在此类杂环的 2 位乙酰化可增强吸电子能力。我们还观察到 1,2,3-二氮杂萘基取代基的电活性,因为它可以在从 -1.97 到 -2.93 V 的电位下与Fc 0/+对不可逆地降低。所有制备的化合物都用光谱方法和的分子结构被完全表征图2a,图2b,图2c,图5a,8,9和10中报告。
更新日期:2021-09-17
中文翻译:

1,2,3-二氮杂萘酚-5-基取代二茂铁的合成及性质
乙酰二茂铁腙或1,1'-二乙酰二茂铁二腙与PCl 3 的反应得到相应的吖嗪作为唯一的有机金属产物。腙功能与 1,4-亚苯基或丙烷-2,2-二基间隔基的分离使得能够制备新的杂环化合物 [(2 H -1,2,3-二氮杂磷-5-基)-4-苯基]二茂铁( 2b )和[1-( 2H -1,2,3-二氮杂磷-5-基)-1-甲基-乙基]二茂铁( 2c )但产率低。使用不会转化为稳定的吖嗪分子的乙酰化腙,合成了(2-乙酰-1,2,3-二氮杂磷-5-基)二茂铁(5a)和1,1'-双(2-)乙酰-1,2,3-二氮杂磷-5-基)二茂铁 ( 8) 的产率高达 70%。N-酰化化合物5a和8很容易转化为 (2 H -1,2,3-二氮杂磷-5-基)二茂铁 ( 2a ) 和 1,1'-双 (2 H -1,2,3-二氮杂磷- 5-基)二茂铁( 9 )。类似的反应用于制备 (2-乙酰基-1,2,3-二氮杂-5-基)二茂铁 ( 10 ), ( 2 H -1,2,3-二氮杂- 5-基)二茂铁 ( 11 ) , 1,1'-双(2-乙酰基-1,2,3-二氮杂-5-基)二茂铁( 12 )和1,1'-双(2 H -1,2,3-二氮杂-5-基) )二茂铁 ( 13 )。一系列新二茂铁的电化学研究表明,2H -1,2,3-diazapnictol-5-yl 取代基具有弱的吸电子能力,通过在此类杂环的 2 位乙酰化可增强吸电子能力。我们还观察到 1,2,3-二氮杂萘基取代基的电活性,因为它可以在从 -1.97 到 -2.93 V 的电位下与Fc 0/+对不可逆地降低。所有制备的化合物都用光谱方法和的分子结构被完全表征图2a,图2b,图2c,图5a,8,9和10中报告。











































 京公网安备 11010802027423号
京公网安备 11010802027423号