Journal of Molecular Graphics and Modelling ( IF 2.7 ) Pub Date : 2021-09-17 , DOI: 10.1016/j.jmgm.2021.108035 Wei Bu Wang 1 , Yu Liang 2 , Yu Qin Jin 2 , Jing Zhang 2 , Ji Guo Su 3 , Qi Ming Li 2
|
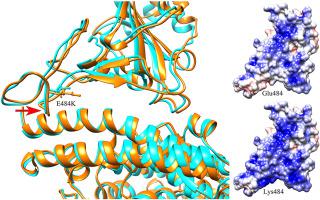
The pandemic of the COVID-19 disease caused by SARS-CoV-2 has led to more than 200 million infections and over 4 million deaths worldwide. The progress in the developments of effective vaccines and neutralizing antibody therapeutics brings hopes to eliminate the threat of COVID-19. However, SARS-CoV-2 continues to mutate, and several new variants have been emerged. Among the various naturally-occurring mutations, the E484K mutation shared by many variants attracted serious concerns, which may potentially enhance the receptor binding affinity and reduce the immune response. In the present study, the molecular mechanism behind the impacts of E484K mutation on the binding affinity of the receptor-binding domain (RBD) with the receptor human angiotensin-converting enzyme 2 (hACE2) was investigated by using the molecular dynamics (MD) simulations combined with the molecular mechanics-generalized Born surface area (MMGBSA) method. Our results indicate that the E484K mutation results in more favorable electrostatic interactions compensating the burial of the charged and polar groups upon the binding of RBD with hACE2, which significantly improves the RBD-hACE2 binding affinity. Besides that, the E484K mutation also causes the conformational rearrangements of the loop region containing the mutant residue, which leads to tighter binding interface of RBD with hACE2 and formation of some new hydrogen bonds. The tighter binding interface and the new hydrogen bonds formation also contribute to the improved binding affinity of RBD to the receptor hACE2. In addition, six neutralizing antibodies and nanobodies complexed with RBD were selected to explore the effects of E484K mutation on the recognition of these antibodies to RBD. The simulation results show that the E484K mutation significantly reduces the binding affinities to RBD for most of the studied neutralizing antibodies/nanobodies, and the decrease in the binding affinities is mainly owing to the unfavorable electrostatic interactions caused by the mutation. Our studies revealed that the E484K mutation may improve the binding affinity between RBD and the receptor hACE2, implying more transmissibility of the E484K-containing variants, and weaken the binding affinities between RBD and the studied neutralizing antibodies/nanobodies, indicating reduced effectiveness of these antibodies/nanobodies. Our results provide valuable information for the effective vaccine development and antibody/nanobody drug design.
中文翻译:

SARS-CoV-2 RBD 中的 E484K 突变增强了与 hACE2 的结合亲和力,但减少了与中和抗体和纳米抗体的相互作用:结合自由能计算研究
由 SARS-CoV-2 引起的 COVID-19 疾病大流行已导致全球超过 2 亿人感染,超过 400 万人死亡。有效疫苗和中和抗体疗法的开发进展为消除 COVID-19 的威胁带来了希望。然而,SARS-CoV-2继续变异,并出现了几种新的变种。在各种自然发生的突变中,许多变体共有的E484K突变引起了人们的严重关注,它可能会增强受体结合亲和力并降低免疫反应。在本研究中,通过分子动力学(MD)模拟研究了E484K突变对受体结合域(RBD)与受体人血管紧张素转换酶2(hACE2)的结合亲和力影响背后的分子机制。结合分子力学-广义玻恩表面积(MMGBSA)方法。我们的结果表明,E484K 突变导致更有利的静电相互作用,补偿 RBD 与 hACE2 结合时带电基团和极性基团的掩埋,从而显着提高 RBD-hACE2 结合亲和力。除此之外,E484K突变还引起含有突变残基的环区构象重排,导致RBD与hACE2的结合界面更紧密,并形成一些新的氢键。更紧密的结合界面和新氢键的形成也有助于提高 RBD 与受体 hACE2 的结合亲和力。此外,还选择了六种中和抗体以及与RBD复合的纳米抗体来探讨E484K突变对这些抗体对RBD识别的影响。模拟结果表明,E484K突变显着降低了大多数所研究的中和抗体/纳米抗体与RBD的结合亲和力,而结合亲和力的降低主要是由于突变引起的不利的静电相互作用。我们的研究表明,E484K 突变可能会提高 RBD 与受体 hACE2 之间的结合亲和力,这意味着含有 E484K 的变体具有更高的传播性,并削弱 RBD 与所研究的中和抗体/纳米抗体之间的结合亲和力,表明这些抗体的有效性降低/纳米抗体。我们的结果为有效的疫苗开发和抗体/纳米抗体药物设计提供了有价值的信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号