Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2021-09-17 , DOI: 10.1016/j.jmb.2021.167254 Sreemantee Sen 1 , Harish Kumar 1 , Jayant B Udgaonkar 1
|
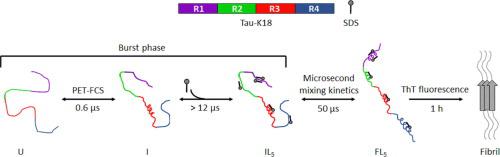
Tau is an intrinsically disordered protein implicated in many neurodegenerative diseases. The repeat domain fragment of tau, tau-K18, is known to undergo a disorder to order transition in the presence of lipid micelles and vesicles, in which helices form in each of the repeat domains. Here, the mechanism of helical structure formation, induced by a phospholipid mimetic, sodium dodecyl sulfate (SDS) at sub-micellar concentrations, has been studied using multiple biophysical probes. A study of the conformational dynamics of the disordered state, using photoinduced electron transfer coupled to fluorescence correlation spectroscopy (PET-FCS) has indicated the presence of an intermediate state, I, in equilibrium with the unfolded state, U. The cooperative binding of the ligand (L), SDS, to I has been shown to induce the formation of a compact, helical intermediate (IL5) within the dead time (∼37 µs) of a continuous flow mixer. Quantitative analysis of the PET-FCS data and the ensemble microsecond kinetic data, suggests that the mechanism of induction of helical structure can be described by a U ↔ I ↔ IL5 ↔ FL5 mechanism, in which the final helical state, FL5, forms from IL5 with a time constant of 50–200 µs. Finally, it has been shown that the helical conformation is an aggregation-competent state that can directly form amyloid fibrils.
中文翻译:

固有无序蛋白质结合诱导折叠过程中的微秒动力学
Tau 是一种内在紊乱的蛋白质,与许多神经退行性疾病有关。已知 tau 的重复结构域片段 tau-K18 在存在脂质胶束和囊泡的情况下会发生有序转变,其中在每个重复结构域中形成螺旋。在这里,已经使用多种生物物理探针研究了由亚胶束浓度的磷脂模拟物十二烷基硫酸钠 (SDS) 诱导的螺旋结构形成机制。使用光致电子转移耦合到荧光相关光谱 (PET-FCS) 对无序状态的构象动力学进行的研究表明,存在与未折叠状态 U 平衡的中间状态 I。配体 (L), SDS, to I 已显示诱导形成一个紧凑的,5 ) 在连续流动混合器的死区时间 (∼37 µs) 内。PET-FCS 数据和集合微秒动力学数据的定量分析表明,螺旋结构的诱导机制可以用 U ↔ I ↔ IL 5 ↔ FL 5机制来描述,其中最终的螺旋状态 FL 5,从 IL 5形成,时间常数为 50–200 µs。最后,已经表明螺旋构象是一种可以直接形成淀粉样原纤维的聚集状态。











































 京公网安备 11010802027423号
京公网安备 11010802027423号