当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel(II)-Catalyzed Addition of Aryl and Heteroaryl Boroxines to the Sulfinylamine Reagent TrNSO: The Catalytic Synthesis of Sulfinamides, Sulfonimidamides, and Primary Sulfonamides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-17 , DOI: 10.1021/jacs.1c08052 Pui Kin Tony Lo 1 , Michael C Willis 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-17 , DOI: 10.1021/jacs.1c08052 Pui Kin Tony Lo 1 , Michael C Willis 1
Affiliation
|
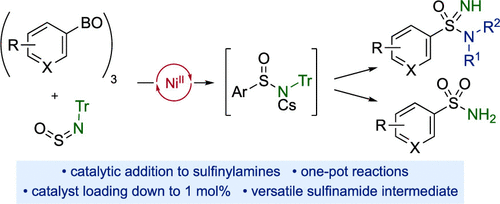
We report a redox-neutral Ni(II)-catalyzed addition of (hetero)aryl boroxines to N-sulfinyltritylamine (TrNSO). The reactions use a catalyst generated from the combination of commercial, air-stable NiCl2·(glyme) and a commercially available bipyridine ligand, and deliver sulfinamide products. The scope of the reaction is established using a sulfonimidamide synthesis, in which the initially formed sulfinamides undergo oxidative chlorination with the inexpensive and safe chlorinating agent, trichloroisocyanuric acid (TCCA), to produce sulfonimidoyl chlorides as key intermediates. These are combined in situ with a range of amines to deliver sulfonimidamides. The sulfonimidoyl chlorides can also be elaborated into primary sulfonamides via hydrolysis, and sulfonimidoyl fluorides via treatment with fluoride. These transformations are all achieved using one-pot procedures. Unprotected, primary sulfinamides are also available. For larger-scale reactions, the catalyst loading can be reduced to 1 mol %.
中文翻译:

镍 (II) - 将芳基和杂芳基环硼氧烷催化加成到亚磺胺试剂 TrNSO:亚磺酰胺、磺酰亚胺和伯磺酰胺的催化合成
我们报告了氧化还原中性 Ni (II) 催化的(杂)芳基环硼氧烷与N-亚磺酰基三苯甲基胺 (TrNSO) 的加成反应。该反应使用由商业、空气稳定的 NiCl 2组合产生的催化剂(Glyme) 和市售的联吡啶配体,并提供亚磺酰胺产品。该反应的范围是使用磺酰亚胺酰胺合成确定的,其中最初形成的亚磺酰胺与廉价且安全的氯化剂三氯异氰尿酸 (TCCA) 进行氧化氯化,以生产磺酰亚胺酰氯作为关键中间体。这些在原位与一系列胺结合以提供磺酰亚胺酰胺。磺酰亚胺酰氯也可以通过水解制成伯磺酰胺,通过氟化物处理制成磺酰亚胺酰氟。这些转化都是使用一锅法实现的。也可以使用未保护的初级亚磺酰胺。对于更大规模的反应,催化剂负载量可以减少到 1 mol%。
更新日期:2021-09-29
中文翻译:

镍 (II) - 将芳基和杂芳基环硼氧烷催化加成到亚磺胺试剂 TrNSO:亚磺酰胺、磺酰亚胺和伯磺酰胺的催化合成
我们报告了氧化还原中性 Ni (II) 催化的(杂)芳基环硼氧烷与N-亚磺酰基三苯甲基胺 (TrNSO) 的加成反应。该反应使用由商业、空气稳定的 NiCl 2组合产生的催化剂(Glyme) 和市售的联吡啶配体,并提供亚磺酰胺产品。该反应的范围是使用磺酰亚胺酰胺合成确定的,其中最初形成的亚磺酰胺与廉价且安全的氯化剂三氯异氰尿酸 (TCCA) 进行氧化氯化,以生产磺酰亚胺酰氯作为关键中间体。这些在原位与一系列胺结合以提供磺酰亚胺酰胺。磺酰亚胺酰氯也可以通过水解制成伯磺酰胺,通过氟化物处理制成磺酰亚胺酰氟。这些转化都是使用一锅法实现的。也可以使用未保护的初级亚磺酰胺。对于更大规模的反应,催化剂负载量可以减少到 1 mol%。











































 京公网安备 11010802027423号
京公网安备 11010802027423号