Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mobility and Reactivity of Cu+ Species in Cu-CHA Catalysts under NH3-SCR-NOx Reaction Conditions: Insights from AIMD Simulations
JACS Au ( IF 8.5 ) Pub Date : 2021-09-16 , DOI: 10.1021/jacsau.1c00337 Reisel Millan 1 , Pieter Cnudde 2 , Veronique van Speybroeck 2 , Mercedes Boronat 1
JACS Au ( IF 8.5 ) Pub Date : 2021-09-16 , DOI: 10.1021/jacsau.1c00337 Reisel Millan 1 , Pieter Cnudde 2 , Veronique van Speybroeck 2 , Mercedes Boronat 1
Affiliation
|
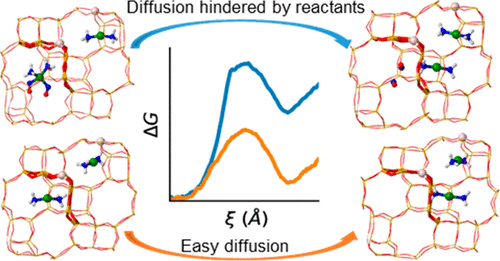
The mobility of the copper cations acting as active sites for the selective catalytic reduction of nitrogen oxides with ammonia in Cu-CHA catalysts varies with temperature and feed composition. Herein, the migration of [Cu(NH3)2]+ complexes between two adjacent cavities of the chabazite structure, including other reactant molecules (NO, O2, H2O, and NH3), in the initial and final cavities is investigated using ab initio molecular dynamics (AIMD) simulations combined with enhanced sampling techniques to describe hopping events from one cage to the other. We find that such diffusion is only significantly hindered by the presence of excess NH3 or NO in the initial cavity, since both reactants form with [Cu(NH3)2]+ stable intermediates which are too bulky to cross the 8-ring windows connecting the cavities. The presence of O2 modifies strongly the interaction of NO with Cu+. At low temperatures, we observe NO detachment from Cu+ and increased mobility of the [Cu(NH3)2]+ complex, while at high temperatures, NO reacts spontaneously with O2 to form NO2. The present simulations give evidence for recent experimental observations, namely, an NH3 inhibition effect on the SCR reaction at low temperatures, and transport limitations of NO and NH3 at high temperatures. Our first principle simulations mimicking operating conditions support the existence of two different reaction mechanisms operating at low and high temperatures, the former involving dimeric Cu(NH3)2-O2-Cu(NH3)2 species and the latter occurring by direct NO oxidation to NO2 in one single cavity.
中文翻译:

在 NH3-SCR-NOx 反应条件下 Cu-CHA 催化剂中 Cu+ 物质的迁移率和反应性:来自 AIMD 模拟的见解
在 Cu-CHA 催化剂中,作为用氨选择性催化还原氮氧化物的活性位点的铜阳离子的迁移率随温度和进料组成而变化。在本文中,[Cu(NH 3 ) 2 ] +复合物在菱沸石结构的两个相邻空腔之间的迁移,包括其他反应物分子(NO、O 2、H 2 O 和 NH 3),在初始和最终空腔中的迁移为使用 ab initio 分子动力学 (AIMD) 模拟结合增强的采样技术来描述从一个笼子到另一个笼子的跳跃事件。我们发现这种扩散仅受到过量 NH 3的存在显着阻碍或初始空腔中的 NO,因为两种反应物都与 [Cu(NH 3 ) 2 ] +稳定的中间体形成,这些中间体体积太大而无法穿过连接空腔的 8 环窗口。O 2的存在极大地改变了NO与Cu +的相互作用。在低温下,我们观察到 NO 与 Cu +分离并增加了 [Cu(NH 3 ) 2 ] +络合物的迁移率,而在高温下,NO 会自发地与 O 2反应形成 NO 2。目前的模拟为最近的实验观察提供了证据,即 NH 3低温下对 SCR 反应的抑制作用,以及高温下 NO 和 NH 3 的传输限制。我们模拟操作条件的第一原理模拟支持在低温和高温下存在两种不同的反应机制,前者涉及二聚体 Cu(NH 3 ) 2 -O 2 -Cu(NH 3 ) 2物种,后者通过直接 NO 发生在一个空腔中氧化成 NO 2。
更新日期:2021-10-25
中文翻译:

在 NH3-SCR-NOx 反应条件下 Cu-CHA 催化剂中 Cu+ 物质的迁移率和反应性:来自 AIMD 模拟的见解
在 Cu-CHA 催化剂中,作为用氨选择性催化还原氮氧化物的活性位点的铜阳离子的迁移率随温度和进料组成而变化。在本文中,[Cu(NH 3 ) 2 ] +复合物在菱沸石结构的两个相邻空腔之间的迁移,包括其他反应物分子(NO、O 2、H 2 O 和 NH 3),在初始和最终空腔中的迁移为使用 ab initio 分子动力学 (AIMD) 模拟结合增强的采样技术来描述从一个笼子到另一个笼子的跳跃事件。我们发现这种扩散仅受到过量 NH 3的存在显着阻碍或初始空腔中的 NO,因为两种反应物都与 [Cu(NH 3 ) 2 ] +稳定的中间体形成,这些中间体体积太大而无法穿过连接空腔的 8 环窗口。O 2的存在极大地改变了NO与Cu +的相互作用。在低温下,我们观察到 NO 与 Cu +分离并增加了 [Cu(NH 3 ) 2 ] +络合物的迁移率,而在高温下,NO 会自发地与 O 2反应形成 NO 2。目前的模拟为最近的实验观察提供了证据,即 NH 3低温下对 SCR 反应的抑制作用,以及高温下 NO 和 NH 3 的传输限制。我们模拟操作条件的第一原理模拟支持在低温和高温下存在两种不同的反应机制,前者涉及二聚体 Cu(NH 3 ) 2 -O 2 -Cu(NH 3 ) 2物种,后者通过直接 NO 发生在一个空腔中氧化成 NO 2。











































 京公网安备 11010802027423号
京公网安备 11010802027423号