当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Axially Chiral Naphthyl-C3-indoles via a Palladium-Catalyzed Cacchi Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.orglett.1c02574 Cong-Shuai Wang 1 , Liang Wei 1 , Cong Fu 1 , Xin-Heng Wang 1 , Chun-Jiang Wang 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2021-09-17 , DOI: 10.1021/acs.orglett.1c02574 Cong-Shuai Wang 1 , Liang Wei 1 , Cong Fu 1 , Xin-Heng Wang 1 , Chun-Jiang Wang 1, 2
Affiliation
|
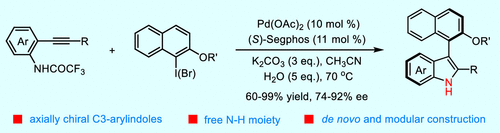
Atropoisomeric biaryl motifs are widely found in natural products and bioactive compounds as well as chiral catalysts and ligands. Various efficient approaches have been disclosed for the construction of chiral six–six biaryl skeletons. In contrast, the enantioselective synthesis of axially chiral arylindoles through the strategy of de novo construction, other than the asymmetric functionalization of indoles, remain a challenging task. Herein we report an efficient Pd(0)/(S)-Segphos-catalyzed atroposelective Cacchi reaction of 2-alkynylanilines with sterically congested naphthyl halides, which afforded an array of naphthyl-C3-indoles in high yields with good to excellent atroposelectivities. The addition of water and the modulation of the manipulation procedure by premixing the palladium complex and the naphthyl halide were the keys to success. The conformational stability of the obtained axially chiral naphthyl-C3-indole containing a synthetically more-valuable free NH moiety is revealed through kinetic experiments.
中文翻译:

通过钯催化的 Cacchi 反应不对称合成轴向手性萘基-C3-吲哚
阻转异构联芳基基序广泛存在于天然产物和生物活性化合物以及手性催化剂和配体中。已经公开了用于构建手性六-六联芳基骨架的各种有效方法。相比之下,除了吲哚的不对称功能化之外,通过从头构建策略对轴向手性芳基吲哚进行对映选择性合成仍然是一项具有挑战性的任务。在这里,我们报告了一个有效的 Pd(0)/( S)-Segphos 催化的 2-炔基苯胺与空间拥挤的萘基卤化物的 atroposelective Cacchi 反应,以高产率提供一系列萘基-C3-吲哚,具有良好到优异的 atroposelectivity。通过预混合钯配合物和萘基卤化物来添加水和调节操作程序是成功的关键。通过动力学实验揭示了所获得的轴向手性萘基-C3-吲哚的构象稳定性,其中含有合成上更有价值的游离 NH 部分。
更新日期:2021-10-01
中文翻译:

通过钯催化的 Cacchi 反应不对称合成轴向手性萘基-C3-吲哚
阻转异构联芳基基序广泛存在于天然产物和生物活性化合物以及手性催化剂和配体中。已经公开了用于构建手性六-六联芳基骨架的各种有效方法。相比之下,除了吲哚的不对称功能化之外,通过从头构建策略对轴向手性芳基吲哚进行对映选择性合成仍然是一项具有挑战性的任务。在这里,我们报告了一个有效的 Pd(0)/( S)-Segphos 催化的 2-炔基苯胺与空间拥挤的萘基卤化物的 atroposelective Cacchi 反应,以高产率提供一系列萘基-C3-吲哚,具有良好到优异的 atroposelectivity。通过预混合钯配合物和萘基卤化物来添加水和调节操作程序是成功的关键。通过动力学实验揭示了所获得的轴向手性萘基-C3-吲哚的构象稳定性,其中含有合成上更有价值的游离 NH 部分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号