当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ring Construction by 1,1-Carboboration; Making Anthracene Derivatives from a Tetra(alkynyl)benzene
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2021-09-15 , DOI: 10.1002/hlca.202100149 Gerhard Erker 1 , René Liedtke 2 , Sabrina Surmiak 3 , Xiaoming Jie 4 , Constantin G. Daniliuc 5 , Gerald Kehr 6
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2021-09-15 , DOI: 10.1002/hlca.202100149 Gerhard Erker 1 , René Liedtke 2 , Sabrina Surmiak 3 , Xiaoming Jie 4 , Constantin G. Daniliuc 5 , Gerald Kehr 6
Affiliation
|
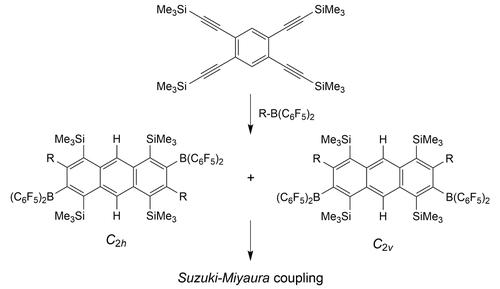
1,2,4,5-Tetrakis(trimethylsilylethynyl)benzene reacted with two molar equivalents of the boranes R−B(C6F5)2 (R=C6F5, Me, Ph) in a series of sequential 1,1-carboboration reactions to give ca. 1 : 1 mixtures of the two-fold benzannulated products, namely the respective C2h and C2v symmetric tetra-silyl, bis-boryl substituted anthracenes. Their active B(C6F5)2 substituents were used for consecutive Suzuki-Miyaura C−C coupling reactions to give boron-free phenyl or 2-pyridyl substituted anthracene products.
中文翻译:

1,1-碳硼化环结构;从四(炔基)苯制备蒽衍生物
1,2,4,5-四(三甲基甲硅烷基乙炔基)苯与两摩尔当量的硼烷 R-B(C 6 F 5 ) 2 (R=C 6 F 5 , Me, Ph) 在一系列顺序 1 中反应, 1-碳化反应得到约。双重苯并环化产物的1:1混合物,即各自的C 2 h和C 2 v对称四甲硅烷基、双硼基取代的蒽。它们的活性 B(C 6 F 5 ) 2取代基用于连续的Suzuki-Miyaura C-C 偶联反应得到无硼苯基或 2-吡啶基取代的蒽产物。
更新日期:2021-11-15
中文翻译:

1,1-碳硼化环结构;从四(炔基)苯制备蒽衍生物
1,2,4,5-四(三甲基甲硅烷基乙炔基)苯与两摩尔当量的硼烷 R-B(C 6 F 5 ) 2 (R=C 6 F 5 , Me, Ph) 在一系列顺序 1 中反应, 1-碳化反应得到约。双重苯并环化产物的1:1混合物,即各自的C 2 h和C 2 v对称四甲硅烷基、双硼基取代的蒽。它们的活性 B(C 6 F 5 ) 2取代基用于连续的Suzuki-Miyaura C-C 偶联反应得到无硼苯基或 2-吡啶基取代的蒽产物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号