当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Poly-Functionalized Indolizines via [5+1] Annulative Access to Pyridines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-09-15 , DOI: 10.1002/adsc.202100844 Dirgha Raj Joshi 1 , Ikyon Kim 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-09-15 , DOI: 10.1002/adsc.202100844 Dirgha Raj Joshi 1 , Ikyon Kim 1
Affiliation
|
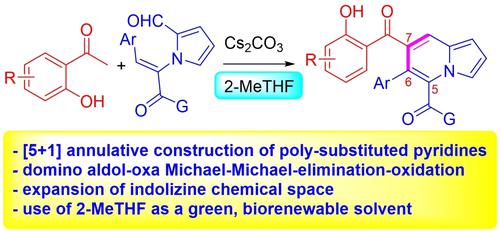
Multi-functionalization of the pyridine motif of indolizine was achieved through [5+1] annulative construction of pyridine from 2-hydroxyacetophenone and pyrrole derivative in the presence of Cs2CO3. The reaction is proposed to take place via domino aldol-oxa Michael-Michael-elimination-oxidation procedure, leading to installation of three different functional groups at the C5, C6, and C7 positions of indolizines. Solvent (2-MeTHF) and the hydroxyl of 2-hydroxyacetophenones seemed to play crucial roles for this successful cyclization event. Further elaboration of the resulting scaffold and expansion of this protocol using other methyl ketones were demonstrated as well.
中文翻译:

通过 [5+1] 环比获得吡啶合成多功能化吲哚嗪
在Cs 2 CO 3存在下,通过由2-羟基苯乙酮和吡咯衍生物[5+1]环状构建吡啶,实现了吲哚嗪的吡啶基序的多功能化。建议该反应通过多米诺醛醇-氧杂迈克尔-迈克尔-消除-氧化过程进行,导致在吲哚嗪的 C5、C6 和 C7 位置安装三个不同的官能团。溶剂 (2-MeTHF) 和 2-羟基苯乙酮的羟基似乎对这一成功的环化事件起着至关重要的作用。还证明了对所得支架的进一步阐述和使用其他甲基酮对本协议的扩展。
更新日期:2021-09-15
中文翻译:

通过 [5+1] 环比获得吡啶合成多功能化吲哚嗪
在Cs 2 CO 3存在下,通过由2-羟基苯乙酮和吡咯衍生物[5+1]环状构建吡啶,实现了吲哚嗪的吡啶基序的多功能化。建议该反应通过多米诺醛醇-氧杂迈克尔-迈克尔-消除-氧化过程进行,导致在吲哚嗪的 C5、C6 和 C7 位置安装三个不同的官能团。溶剂 (2-MeTHF) 和 2-羟基苯乙酮的羟基似乎对这一成功的环化事件起着至关重要的作用。还证明了对所得支架的进一步阐述和使用其他甲基酮对本协议的扩展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号