Chinese Journal of Catalysis ( IF 15.7 ) Pub Date : 2021-09-15 , DOI: 10.1016/s1872-2067(21)64088-3 Yang Qiu 1 , Xiaohong Xie 1 , Wenzhen Li 2, 3 , Yuyan Shao 1
|
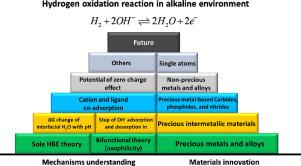
Anion exchange membrane (AEM) fuel cells have gained great attention partially due to the advantage of using non-precious metal as catalysts. However, the reaction kinetics of hydrogen oxidation reaction (HOR) is two orders of magnitude slower in alkaline systems than in acid. To understand the slower kinetics of HOR in base, two major theories have been proposed, such as (1) pH dependent hydrogen binding energy as a major descriptor for HOR; and (2) bifunctional theory based on the contributions of both hydrogen and hydroxide adsorption for HOR in alkaline electrolyte. Here, we discuss the possible HOR mechanisms in alkaline electrolytes with the corresponding change in their Tafel behavior. Apart from the traditional Tafel-Volmer and Heyrovsky-Volmer HOR mechanisms, the recently proposed hydroxide adsorption step is also discussed to illustrate the difference in HOR mechanisms in acid and base. We further summarize the representative works of alkaline HOR catalyst design (e.g., precious metals, alloy, intermetallic materials, Ni-based alloys, carbides, nitrides, etc.), and briefly describe their fundamental HOR reaction mechanism to emphasize the difference in elementary reaction steps in alkaline medium. The strategy of strengthening local interaction that facilitates both H2 desorption and Hads + OHads recombination is finally proposed for future HOR catalyst design in alkaline environment.
中文翻译:

碱性介质中氢氧化反应的电催化剂开发:从机理理解到材料设计
阴离子交换膜(AEM)燃料电池因其使用非贵金属作为催化剂的优势而备受关注。然而,氢氧化反应 (HOR) 的反应动力学在碱性系统中比在酸系统中慢两个数量级。为了理解碱中 HOR 较慢的动力学,已经提出了两个主要理论,例如(1)pH 依赖性氢结合能作为 HOR 的主要描述;(2) 基于氢和氢氧化物吸附对碱性电解液中 HOR 的贡献的双功能理论。在这里,我们讨论了碱性电解质中可能的 HOR 机制及其 Tafel 行为的相应变化。除了传统的 Tafel-Volmer 和 Heyrovsky-Volmer HOR 机制,还讨论了最近提出的氢氧化物吸附步骤,以说明酸和碱中 HOR 机制的差异。我们进一步总结了碱性HOR催化剂设计的代表性作品(如贵金属、合金、金属间材料、镍基合金、碳化物、氮化物等),并简要描述了它们的基本HOR反应机理,以强调基元反应的差异碱性介质中的步骤。加强本地互动的战略有利于 H 并简要描述它们的基本 HOR 反应机理,以强调碱性介质中基本反应步骤的差异。加强本地互动的战略有利于 H 并简要描述它们的基本 HOR 反应机理,以强调碱性介质中基本反应步骤的差异。加强本地互动的战略有利于 H2解吸和 H ads + OH ads重组最终被提出用于未来碱性环境下的 HOR 催化剂设计。









































 京公网安备 11010802027423号
京公网安备 11010802027423号