Cell Reports Physical Science ( IF 7.9 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.xcrp.2021.100577 Tong-De Tan 1 , Tong-Yi Zhai 1 , Bin-Yang Liu 1 , Long Li 2 , Peng-Cheng Qian 2 , Qing Sun 3 , Jin-Mei Zhou 1 , Long-Wu Ye 1, 4
|
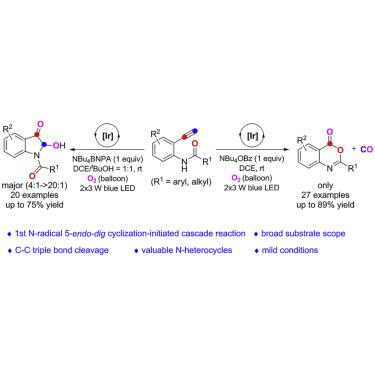
In recent years, significant advances have been made on photoredox-catalyzed N-radical addition to C-C unsaturated bond via N-H bond cleavage. However, these N-centered radical additions have so far been mostly limited to the alkenes, and to our knowledge, N-radical 5-endo-dig cyclizations of alkynes have been less exploited. Herein, we disclose an 5-endo-dig N-radical cascade cyclization of o-ethynylacetamides by photoredox catalysis involving rare radical cleavage of C-C triple bond, allowing the practical and controllable synthesis of a diverse array of valuable benzoxazinones and 2-hydroxy-3-indolinones in moderate to good yields under mild conditions. Furthermore, a mechanistic rationale for this radical cyclization cascade is supported by various control experiments and theoretical calculations.
中文翻译:

通过可见光促进的 5-endo-dig N-自由基环化级联可控合成苯并嗪酮和 2-羟基-3-吲哚酮
近年来,光氧化还原催化的 N-自由基通过 NH 键断裂加成到 CC 不饱和键上取得了重大进展。然而,到目前为止,这些以 N 为中心的自由基加成主要限于烯烃,据我们所知,炔烃的N-自由基 5 -end-dig环化很少被利用。在此,我们公开了o的 5 -endo-dig N-自由基级联环化-乙炔基乙酰胺通过光氧化还原催化涉及 CC 三键的罕见自由基裂解,允许在温和条件下以中等至良好的产率实际且可控地合成各种有价值的苯并嗪酮和 2-羟基-3-吲哚酮。此外,这种自由基环化级联的机械原理得到了各种控制实验和理论计算的支持。










































 京公网安备 11010802027423号
京公网安备 11010802027423号