Current Pharmaceutical Analysis ( IF 0.7 ) Pub Date : 2021-11-30 , DOI: 10.2174/1573412917999201102211941 Sang-Ho Lee 1 , Sang-Ho Lee 2 , Jong-Hyuk Lee 3 , Joo-Eun Kim 2
|
Background: Telmisartan and rosuvastatin calcium fixed-dose combination double-layer tablet is used for hypertension and hyperlipidemia treatment. Owing to the critical problem in establishing the specificity, precision, and accuracy of the USP analysis method and simultaneous analysis method for both components, an analysis method that could be applied to researchers in the field is urgently needed.
Objective: To develop and validate a reversed phase-high-performance liquid chromatography method for the quantitative analysis of dissolution samples of telmisartan and rosuvastatin calcium fixed-dose combination double-layer tablets, as a hypertension and hyperlipidemia treatment.
Methods: The developed analysis method was validated according to USP Category I requirements. The validity of the quantitative assay of the dissolution test solution was determined based on the system suitability, specificity, linearity, accuracy, precision, and solution stability of the assay for the components.
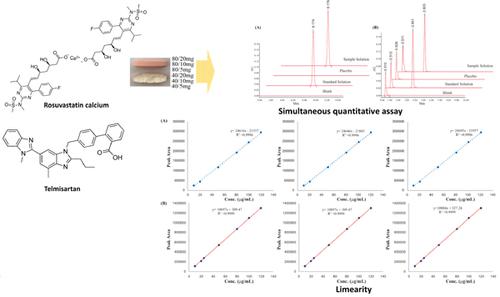
Results: The developed method was selective and precise. The retention times of telmisartan and rosuvastatin calcium was 8.1 min and 2.8 min, respectively, and system suitability was 0.217 and 0.17, respectively. Both components showed linearity at 10–120% concentration. The accuracy and precision were also within acceptable limits. The drugs in the dissolution samples were stable for a long time, no interaction occurred between the excipients and active pharmaceutical ingredients, and no interference was observed in the analysis.
Conclusion: The quantitative analysis of the double-layered formulation of telmisartan and rosuvastatin calcium was accurate, selective, and precise. Therefore, this method can be recommended for use in the industrial quality control of telmisartan and rosuvastatin calcium formulations.
中文翻译:

用于分析替米沙坦和瑞舒伐他汀钙双层制剂溶出样品的高效液相色谱方法的开发、验证和应用
背景:替米沙坦瑞舒伐他汀钙固定剂量复方双层片用于高血压和高脂血症的治疗。由于在建立 USP 分析方法和同时分析方法对两种组分的特异性、精密度和准确度方面存在关键问题,迫切需要一种可应用于该领域研究人员的分析方法。
目的:开发并验证一种用于定量分析替米沙坦和瑞舒伐他汀钙固定剂量复方双层片溶出样品的反相-高效液相色谱方法,用于高血压和高脂血症的治疗。
方法:根据 USP 类别 I 要求验证开发的分析方法。溶出试液定量测定的有效性取决于组分测定的系统适用性、特异性、线性、准确度、精密度和溶液稳定性。
结果:所开发的方法具有选择性和精确性。替米沙坦和瑞舒伐他汀钙的保留时间分别为 8.1 分钟和 2.8 分钟,系统适用性分别为 0.217 和 0.17。两种组分在 10-120% 浓度下均显示线性。准确度和精密度也在可接受的范围内。溶出样品中药物长期稳定,辅料与药物活性成分之间未发生相互作用,分析中未观察到干扰。
结论:替米沙坦和瑞舒伐他汀钙双层制剂定量分析准确、选择性、精密。因此,该方法可推荐用于替米沙坦和瑞舒伐他汀钙制剂的工业质量控制。









































 京公网安备 11010802027423号
京公网安备 11010802027423号