当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal–Organic Framework-Based Nanoagents for Effective Tumor Therapy by Dual Dynamics-Amplified Oxidative Stress
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-09-16 , DOI: 10.1021/acsami.1c11032 Jiajie Chen 1, 2, 3 , Yitong Wang 1 , Huicong Niu 3 , Yuwei Wang 4 , Aijun Wu 3, 4 , Chaoqin Shu 3, 4 , Yufang Zhu 2, 3 , Yuhai Bian 5 , Kaili Lin 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-09-16 , DOI: 10.1021/acsami.1c11032 Jiajie Chen 1, 2, 3 , Yitong Wang 1 , Huicong Niu 3 , Yuwei Wang 4 , Aijun Wu 3, 4 , Chaoqin Shu 3, 4 , Yufang Zhu 2, 3 , Yuhai Bian 5 , Kaili Lin 1
Affiliation
|
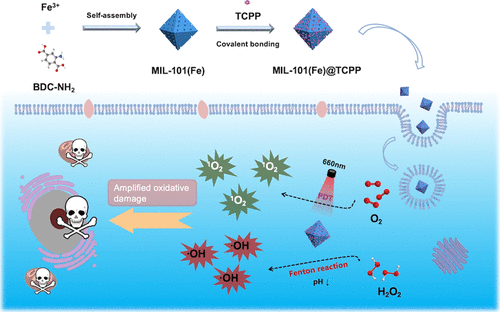
Overproduction of reactive oxygen species (ROS) within tumors can cause oxidative stress on tumor cells to induce death, which has motivated us to develop ROS-mediated tumor therapies, such as typical photodynamic therapy (PDT) and Fenton reaction-mediated chemodynamic therapy (CDT). However, these therapeutic modalities suffer from compromised treatment efficacy owing to their limited generation of highly reactive ROS in a tumor microenvironment (TME). In this work, a nanoscale iron-based metal–organic framework, MIL-101(Fe), is synthesized as a Fenton nanocatalyst to perform the catalytic conversion of hydroxyl radicals (·OH) from hydrogen peroxide (H2O2) under the acidic environment and as a biocompatible and biodegradable nanocarrier to deliver a 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin (TCPP) photosensitizer for light-activated singlet oxygen (1O2) generation. By coupling such chemodynamic/photodynamic effects, the photosensitizer-integrated nanoagents (MIL-101(Fe)@TCPP) could enable more ROS production within tumors to induce amplified oxidative damage for tumor-specific synergistic therapy. In vitro results show that MIL-101(Fe)@TCPP nanoagents achieve the acid-responsive CDT and effective PDT, and synergistic CDT/PDT provides an enhanced therapeutic effect. Ultimately, based on such synergistic therapy, MIL-101(Fe)@TCPP nanoagents cause a significant tumor growth inhibition in vivo without severe side effects, showing great potential for anti-tumor application.
中文翻译:

基于金属有机框架的纳米药物通过双动力学放大氧化应激有效治疗肿瘤
肿瘤内活性氧(ROS)的过量产生会导致肿瘤细胞的氧化应激诱导死亡,这促使我们开发ROS介导的肿瘤疗法,例如典型的光动力疗法(PDT)和芬顿反应介导的化学动力学疗法(CDT) )。然而,由于它们在肿瘤微环境(TME)中产生的高反应活性氧有限,这些治疗方式的治疗效果受到影响。在这项工作中,合成了一种纳米级铁基金属有机骨架 MIL-101(Fe) 作为 Fenton 纳米催化剂,用于从过氧化氢 (H 2 O 2 ) 催化转化羟基自由基 (·OH)) 在酸性环境下作为生物相容性和可生物降解的纳米载体提供 5,10,15,20-四(4-羧基苯基)卟啉 (TCPP) 光敏剂,用于光活化单线态氧 ( 1 O 2 ) 生成。通过耦合这种化学动力学/光动力学效应,光敏剂集成纳米剂 (MIL-101(Fe)@TCPP) 可以使肿瘤内产生更多的 ROS,从而诱导肿瘤特异性协同治疗的放大氧化损伤。体外实验结果表明,MIL-101(Fe)@TCPP纳米制剂实现了酸响应性CDT和有效的PDT,协同CDT/PDT提供了增强的治疗效果。最终,基于这种协同治疗,MIL-101(Fe)@TCPP 纳米制剂在体内引起显着的肿瘤生长抑制 无严重副作用,显示出巨大的抗肿瘤应用潜力。
更新日期:2021-09-29
中文翻译:

基于金属有机框架的纳米药物通过双动力学放大氧化应激有效治疗肿瘤
肿瘤内活性氧(ROS)的过量产生会导致肿瘤细胞的氧化应激诱导死亡,这促使我们开发ROS介导的肿瘤疗法,例如典型的光动力疗法(PDT)和芬顿反应介导的化学动力学疗法(CDT) )。然而,由于它们在肿瘤微环境(TME)中产生的高反应活性氧有限,这些治疗方式的治疗效果受到影响。在这项工作中,合成了一种纳米级铁基金属有机骨架 MIL-101(Fe) 作为 Fenton 纳米催化剂,用于从过氧化氢 (H 2 O 2 ) 催化转化羟基自由基 (·OH)) 在酸性环境下作为生物相容性和可生物降解的纳米载体提供 5,10,15,20-四(4-羧基苯基)卟啉 (TCPP) 光敏剂,用于光活化单线态氧 ( 1 O 2 ) 生成。通过耦合这种化学动力学/光动力学效应,光敏剂集成纳米剂 (MIL-101(Fe)@TCPP) 可以使肿瘤内产生更多的 ROS,从而诱导肿瘤特异性协同治疗的放大氧化损伤。体外实验结果表明,MIL-101(Fe)@TCPP纳米制剂实现了酸响应性CDT和有效的PDT,协同CDT/PDT提供了增强的治疗效果。最终,基于这种协同治疗,MIL-101(Fe)@TCPP 纳米制剂在体内引起显着的肿瘤生长抑制 无严重副作用,显示出巨大的抗肿瘤应用潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号