当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
p38 MAPK-mediated loss of nuclear RNase III enzyme Drosha underlies amyloid beta-induced neuronal stress in Alzheimer's disease
Aging Cell ( IF 8.0 ) Pub Date : 2021-09-16 , DOI: 10.1111/acel.13434 Haidong Xu 1 , Xiaolei Liu 1 , Wenming Li 1 , Ye Xi 1 , Peng Su 1 , Bo Meng 1 , Xiaoyun Shao 1 , Beisha Tang 2 , Qian Yang 3 , Zixu Mao 1, 4
Aging Cell ( IF 8.0 ) Pub Date : 2021-09-16 , DOI: 10.1111/acel.13434 Haidong Xu 1 , Xiaolei Liu 1 , Wenming Li 1 , Ye Xi 1 , Peng Su 1 , Bo Meng 1 , Xiaoyun Shao 1 , Beisha Tang 2 , Qian Yang 3 , Zixu Mao 1, 4
Affiliation
|
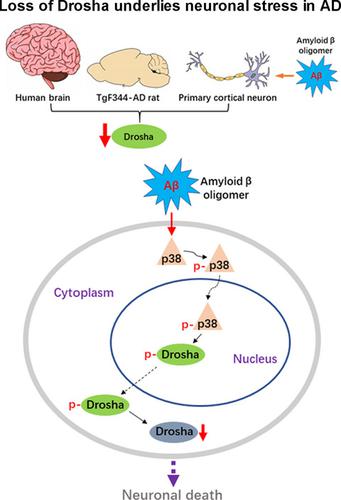
MicroRNAs (miRNAs) are small noncoding RNAs ubiquitously expressed in the brain and regulate gene expression at the post-transcriptional level. The nuclear RNase III enzyme Drosha initiates the maturation process of miRNAs in the nucleus. Strong evidence suggests that dysregulation of miRNAs is involved in many neurological disorders including Alzheimer's disease (AD). Dysfunction of miRNA biogenesis components may be involved in the processes of those diseases. However, the role of Drosha in AD remains unknown. By using immunohistochemistry, biochemistry, and subcellular fractionation methods, we show here that the level of Drosha protein was significantly lower in the postmortem brain of human AD patients as well as in the transgenic rat model of AD. Interestingly, Drosha level was specifically reduced in neurons of the cortex and hippocampus but not in the cerebellum in the AD brain samples. In primary cortical neurons, amyloid-beta (Aβ) oligomers caused a p38 MAPK-dependent phosphorylation of Drosha, leading to its redistribution from the nucleus to the cytoplasm and a decrease in its level. This loss of Drosha function preceded Aβ-induced neuronal death. Importantly, inhibition of p38 MAPK activity or overexpression of Drosha protected neurons from Aβ oligomers-induced apoptosis. Taken together, these results establish a role for p38 MAPK-Drosha pathway in modulating neuronal viability under Aβ oligomers stress condition and implicate loss of Drosha as a key molecular change in the pathogenesis of AD.
中文翻译:

p38 MAPK 介导的核 RNase III 酶 Drosha 缺失是淀粉样蛋白 β 诱导的阿尔茨海默病神经元应激的基础
微小RNA(miRNA)是在大脑中普遍表达的小型非编码RNA,并在转录后水平调节基因表达。核 RNase III 酶 Drosha 启动核内 miRNA 的成熟过程。强有力的证据表明,miRNA 的失调与许多神经系统疾病有关,包括阿尔茨海默病 (AD)。miRNA生物发生成分的功能障碍可能与这些疾病的过程有关。然而,Drosha 在 AD 中的作用仍然未知。通过使用免疫组织化学、生物化学和亚细胞分离方法,我们在此显示人类 AD 患者死后大脑以及 AD 转基因大鼠模型中的 Drosha 蛋白水平显着降低。有趣的是,在 AD 脑样本中,Drosha 水平在皮层和海马的神经元中特别降低,但在小脑中没有。在初级皮层神经元中,β-淀粉样蛋白 (Aβ) 寡聚体引起 Drosha 的 p38 MAPK 依赖性磷酸化,导致其从细胞核重新分布到细胞质并降低其水平。这种 Drosha 功能的丧失先于 Aβ 诱导的神经元死亡。重要的是,抑制 p38 MAPK 活性或 Drosha 过表达保护神经元免受 Aβ 寡聚体诱导的细胞凋亡。总之,这些结果确立了 p38 MAPK-Drosha 通路在 Aβ 寡聚体应激条件下调节神经元活力的作用,并暗示 Drosha 的丧失是 AD 发病机制中的关键分子变化。淀粉样蛋白-β (Aβ) 寡聚体引起 Drosha 的 p38 MAPK 依赖性磷酸化,导致其从细胞核重新分布到细胞质并降低其水平。这种 Drosha 功能的丧失先于 Aβ 诱导的神经元死亡。重要的是,抑制 p38 MAPK 活性或 Drosha 过表达保护神经元免受 Aβ 寡聚体诱导的细胞凋亡。总之,这些结果确立了 p38 MAPK-Drosha 通路在 Aβ 寡聚体应激条件下调节神经元活力的作用,并暗示 Drosha 的丧失是 AD 发病机制中的关键分子变化。淀粉样蛋白-β (Aβ) 寡聚体引起 Drosha 的 p38 MAPK 依赖性磷酸化,导致其从细胞核重新分布到细胞质并降低其水平。这种 Drosha 功能的丧失先于 Aβ 诱导的神经元死亡。重要的是,抑制 p38 MAPK 活性或 Drosha 过表达保护神经元免受 Aβ 寡聚体诱导的细胞凋亡。总之,这些结果确立了 p38 MAPK-Drosha 通路在 Aβ 寡聚体应激条件下调节神经元活力的作用,并暗示 Drosha 的丧失是 AD 发病机制中的关键分子变化。抑制 p38 MAPK 活性或 Drosha 过表达保护神经元免受 Aβ 寡聚体诱导的细胞凋亡。总之,这些结果确立了 p38 MAPK-Drosha 通路在 Aβ 寡聚体应激条件下调节神经元活力的作用,并暗示 Drosha 的丧失是 AD 发病机制中的关键分子变化。抑制 p38 MAPK 活性或 Drosha 过表达保护神经元免受 Aβ 寡聚体诱导的细胞凋亡。总之,这些结果确立了 p38 MAPK-Drosha 通路在 Aβ 寡聚体应激条件下调节神经元活力的作用,并暗示 Drosha 的丧失是 AD 发病机制中的关键分子变化。
更新日期:2021-10-17
中文翻译:

p38 MAPK 介导的核 RNase III 酶 Drosha 缺失是淀粉样蛋白 β 诱导的阿尔茨海默病神经元应激的基础
微小RNA(miRNA)是在大脑中普遍表达的小型非编码RNA,并在转录后水平调节基因表达。核 RNase III 酶 Drosha 启动核内 miRNA 的成熟过程。强有力的证据表明,miRNA 的失调与许多神经系统疾病有关,包括阿尔茨海默病 (AD)。miRNA生物发生成分的功能障碍可能与这些疾病的过程有关。然而,Drosha 在 AD 中的作用仍然未知。通过使用免疫组织化学、生物化学和亚细胞分离方法,我们在此显示人类 AD 患者死后大脑以及 AD 转基因大鼠模型中的 Drosha 蛋白水平显着降低。有趣的是,在 AD 脑样本中,Drosha 水平在皮层和海马的神经元中特别降低,但在小脑中没有。在初级皮层神经元中,β-淀粉样蛋白 (Aβ) 寡聚体引起 Drosha 的 p38 MAPK 依赖性磷酸化,导致其从细胞核重新分布到细胞质并降低其水平。这种 Drosha 功能的丧失先于 Aβ 诱导的神经元死亡。重要的是,抑制 p38 MAPK 活性或 Drosha 过表达保护神经元免受 Aβ 寡聚体诱导的细胞凋亡。总之,这些结果确立了 p38 MAPK-Drosha 通路在 Aβ 寡聚体应激条件下调节神经元活力的作用,并暗示 Drosha 的丧失是 AD 发病机制中的关键分子变化。淀粉样蛋白-β (Aβ) 寡聚体引起 Drosha 的 p38 MAPK 依赖性磷酸化,导致其从细胞核重新分布到细胞质并降低其水平。这种 Drosha 功能的丧失先于 Aβ 诱导的神经元死亡。重要的是,抑制 p38 MAPK 活性或 Drosha 过表达保护神经元免受 Aβ 寡聚体诱导的细胞凋亡。总之,这些结果确立了 p38 MAPK-Drosha 通路在 Aβ 寡聚体应激条件下调节神经元活力的作用,并暗示 Drosha 的丧失是 AD 发病机制中的关键分子变化。淀粉样蛋白-β (Aβ) 寡聚体引起 Drosha 的 p38 MAPK 依赖性磷酸化,导致其从细胞核重新分布到细胞质并降低其水平。这种 Drosha 功能的丧失先于 Aβ 诱导的神经元死亡。重要的是,抑制 p38 MAPK 活性或 Drosha 过表达保护神经元免受 Aβ 寡聚体诱导的细胞凋亡。总之,这些结果确立了 p38 MAPK-Drosha 通路在 Aβ 寡聚体应激条件下调节神经元活力的作用,并暗示 Drosha 的丧失是 AD 发病机制中的关键分子变化。抑制 p38 MAPK 活性或 Drosha 过表达保护神经元免受 Aβ 寡聚体诱导的细胞凋亡。总之,这些结果确立了 p38 MAPK-Drosha 通路在 Aβ 寡聚体应激条件下调节神经元活力的作用,并暗示 Drosha 的丧失是 AD 发病机制中的关键分子变化。抑制 p38 MAPK 活性或 Drosha 过表达保护神经元免受 Aβ 寡聚体诱导的细胞凋亡。总之,这些结果确立了 p38 MAPK-Drosha 通路在 Aβ 寡聚体应激条件下调节神经元活力的作用,并暗示 Drosha 的丧失是 AD 发病机制中的关键分子变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号