当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of angiotensin-converting enzyme 2/angiotensin (1–7)/mas receptor axis triggers autophagy and suppresses microglia proinflammatory polarization via forkhead box class O1 signaling
Aging Cell ( IF 8.0 ) Pub Date : 2021-09-16 , DOI: 10.1111/acel.13480 Ruili Dang 1 , Mengqi Yang 1 , Changmeng Cui 2 , Changshui Wang 2 , Wenyuan Zhang 3 , Chunmei Geng 1 , Wenxiu Han 1 , Pei Jiang 1
Aging Cell ( IF 8.0 ) Pub Date : 2021-09-16 , DOI: 10.1111/acel.13480 Ruili Dang 1 , Mengqi Yang 1 , Changmeng Cui 2 , Changshui Wang 2 , Wenyuan Zhang 3 , Chunmei Geng 1 , Wenxiu Han 1 , Pei Jiang 1
Affiliation
|
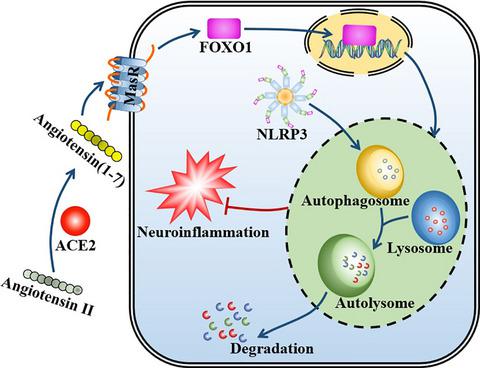
Brain renin-angiotensin (Ang) system (RAS) is implicated in neuroinflammation, a major characteristic of aging process. Angiotensin (Ang) II, produced by angiotensin-converting enzyme (ACE), activates immune system via angiotensin type 1 receptor (AT1), whereas Ang(1–7), generated by ACE2, binds with Mas receptor (MasR) to restrain excessive inflammatory response. Therefore, the present study aims to explore the relationship between RAS and neuroinflammation. We found that repeated lipopolysaccharide (LPS) treatment shifted the balance between ACE/Ang II/AT1 and ACE2/Ang(1–7)/MasR axis to the deleterious side and treatment with either MasR agonist, AVE0991 (AVE) or ACE2 activator, diminazene aceturate, exhibited strong neuroprotective actions. Mechanically, activation of ACE2/Ang(1–7)/MasR axis triggered the Forkhead box class O1 (FOXO1)-autophagy pathway and induced superoxide dismutase (SOD) and catalase (CAT), the FOXO1-targeted antioxidant enzymes. Meanwhile, knockdown of MasR or FOXO1 in BV2 cells, or using the selective FOXO1 inhibitor, AS1842856, in animals, suppressed FOXO1 translocation and compromised the autophagic process induced by MasR activation. We further used chloroquine (CQ) to block autophagy and showed that suppressing either FOXO1 or autophagy abrogated the anti-inflammatory action of AVE. Likewise, Ang(1–7) also induced FOXO1 signaling and autophagic flux following LPS treatment in BV2 cells. Cotreatment with AS1842856 or CQ all led to autophagic inhibition and thereby abolished Ang(1–7)-induced remission on NLRP3 inflammasome activation caused by LPS exposure, shifting the microglial polarization from M1 to M2 phenotype. Collectively, these results firstly illustrated the mechanism of ACE2/Ang(1–7)/MasR axis in neuroinflammation, strongly indicating the involvement of FOXO1-mediated autophagy in the neuroimmune-modulating effects triggered by MasR activation.
中文翻译:

血管紧张素转换酶 2/血管紧张素 (1-7)/mas 受体轴的激活通过叉头盒 O1 类信号传导触发自噬并抑制小胶质细胞促炎极化
脑肾素-血管紧张素 (Ang) 系统 (RAS) 与神经炎症有关,这是衰老过程的主要特征。由血管紧张素转换酶 (ACE) 产生的血管紧张素 (Ang) II 通过血管紧张素 1 型受体 (AT1) 激活免疫系统,而由 ACE2 产生的 Ang(1-7) 与 Mas 受体 (MasR) 结合以抑制过度炎症反应。因此,本研究旨在探讨RAS与神经炎症之间的关系。我们发现重复的脂多糖 (LPS) 治疗将 ACE/Ang II/AT1 和 ACE2/Ang(1-7)/MasR 轴之间的平衡转移到有害的一侧,并使用 MasR 激动剂 AVE0991 (AVE) 或 ACE2 激活剂进行治疗, diminazene aceturate,表现出强烈的神经保护作用。机械地,ACE2/Ang(1-7)/MasR 轴的激活触发了 Forkhead box class O1 (FOXO1)-自噬途径并诱导了 FOXO1 靶向的抗氧化酶超氧化物歧化酶 (SOD) 和过氧化氢酶 (CAT)。同时,在 BV2 细胞中敲除 MasR 或 FOXO1,或在动物中使用选择性 FOXO1 抑制剂 AS1842856,可抑制 FOXO1 易位并损害由 MasR 激活诱导的自噬过程。我们进一步使用氯喹 (CQ) 来阻断自噬,并表明抑制 FOXO1 或自噬会消除 AVE 的抗炎作用。同样,在 BV2 细胞中 LPS 处理后,Ang(1-7) 也诱导 FOXO1 信号传导和自噬通量。与 AS1842856 或 CQ 共同治疗均导致自噬抑制,从而消除了 Ang(1-7) 诱导的 LPS 暴露引起的 NLRP3 炎性体活化缓解,将小胶质细胞极化从 M1 转变为 M2 表型。总的来说,这些结果首先说明了 ACE2/Ang(1-7)/MasR 轴在神经炎症中的作用机制,强烈表明 FOXO1 介导的自噬参与了由 MasR 激活引发的神经免疫调节作用。
更新日期:2021-10-17
中文翻译:

血管紧张素转换酶 2/血管紧张素 (1-7)/mas 受体轴的激活通过叉头盒 O1 类信号传导触发自噬并抑制小胶质细胞促炎极化
脑肾素-血管紧张素 (Ang) 系统 (RAS) 与神经炎症有关,这是衰老过程的主要特征。由血管紧张素转换酶 (ACE) 产生的血管紧张素 (Ang) II 通过血管紧张素 1 型受体 (AT1) 激活免疫系统,而由 ACE2 产生的 Ang(1-7) 与 Mas 受体 (MasR) 结合以抑制过度炎症反应。因此,本研究旨在探讨RAS与神经炎症之间的关系。我们发现重复的脂多糖 (LPS) 治疗将 ACE/Ang II/AT1 和 ACE2/Ang(1-7)/MasR 轴之间的平衡转移到有害的一侧,并使用 MasR 激动剂 AVE0991 (AVE) 或 ACE2 激活剂进行治疗, diminazene aceturate,表现出强烈的神经保护作用。机械地,ACE2/Ang(1-7)/MasR 轴的激活触发了 Forkhead box class O1 (FOXO1)-自噬途径并诱导了 FOXO1 靶向的抗氧化酶超氧化物歧化酶 (SOD) 和过氧化氢酶 (CAT)。同时,在 BV2 细胞中敲除 MasR 或 FOXO1,或在动物中使用选择性 FOXO1 抑制剂 AS1842856,可抑制 FOXO1 易位并损害由 MasR 激活诱导的自噬过程。我们进一步使用氯喹 (CQ) 来阻断自噬,并表明抑制 FOXO1 或自噬会消除 AVE 的抗炎作用。同样,在 BV2 细胞中 LPS 处理后,Ang(1-7) 也诱导 FOXO1 信号传导和自噬通量。与 AS1842856 或 CQ 共同治疗均导致自噬抑制,从而消除了 Ang(1-7) 诱导的 LPS 暴露引起的 NLRP3 炎性体活化缓解,将小胶质细胞极化从 M1 转变为 M2 表型。总的来说,这些结果首先说明了 ACE2/Ang(1-7)/MasR 轴在神经炎症中的作用机制,强烈表明 FOXO1 介导的自噬参与了由 MasR 激活引发的神经免疫调节作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号