Journal of Pharmaceutical Analysis ( IF 6.1 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.jpha.2021.09.006 Deepa Raghu 1 , Pamela Hamill 2 , Arpitha Banaji 1 , Amy McLaren 2 , Yu-Ting Hsu 2, 3
|
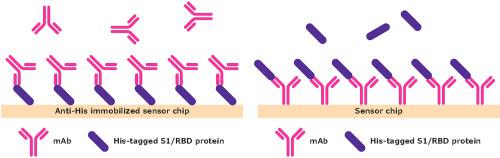
Severe acute respiratory syndrome-associated coronavirus 2 is a major global health issue and is driving the need for new therapeutics. The surface spike protein, which plays a central role in virus infection, is currently the target for vaccines and neutralizing treatments. The emergence of novel variants with multiple mutations in the spike protein may reduce the effectiveness of neutralizing antibodies by altering the binding activity of the protein with angiotensin-converting enzyme 2 (ACE2). To understand the impact of spike protein mutations on the binding interactions required for virus infection and the effectiveness of neutralizing monoclonal antibody (mAb) therapies, the binding activities of the original spike protein receptor binding domain (RBD) sequence and the reported spike protein variants were investigated using surface plasmon resonance. In addition, the interactions of the ACE2 receptor, an anti-spike mAb (mAb1), a neutralizing mAb (mAb2), the original spike RBD sequence, and mutants D614G, N501Y, N439K, Y453F, and E484K were assessed. Compared to the original RBD, the Y453F and N501Y mutants displayed a significant increase in ACE2 binding affinity, whereas D614G had a substantial reduction in binding affinity. All mAb-RBD mutant proteins displayed a reduction in binding affinities relative to the original RBD, except for the E484K-mAb1 interaction. The potential neutralizing capability of mAb1 and mAb2 was investigated. Accordingly, mAb1 failed to inhibit the ACE2-RBD interaction while mAb2 inhibited the ACE2-RBD interactions for all RBD mutants, except mutant E484K, which only displayed partial blocking.
中文翻译:

评估 SARS-CoV-2 刺突糖蛋白变体的结合相互作用
严重急性呼吸综合征相关冠状病毒 2 是一个主要的全球健康问题,正在推动对新疗法的需求。在病毒感染中起核心作用的表面刺突蛋白目前是疫苗和中和治疗的目标。刺突蛋白中具有多个突变的新变体的出现可能会通过改变蛋白质与血管紧张素转换酶 2 (ACE2) 的结合活性来降低中和抗体的有效性。为了了解刺突蛋白突变对病毒感染所需的结合相互作用的影响以及中和单克隆抗体 (mAb) 疗法的有效性,使用表面等离子体共振研究了原始刺突蛋白受体结合域 (RBD) 序列和报告的刺突蛋白变体的结合活性。此外,还评估了 ACE2 受体、抗刺突 mAb (mAb1)、中和 mAb (mAb2)、原始刺突 RBD 序列以及突变体 D614G、N501Y、N439K、Y453F 和 E484K 的相互作用。与原始 RBD 相比,Y453F 和 N501Y 突变体显示出 ACE2 结合亲和力显着增加,而 D614G 结合亲和力显着降低。除 E484K-mAb1 相互作用外,所有 mAb-RBD 突变蛋白均显示出与原始 RBD 的结合亲和力降低。研究了 mAb1 和 mAb2 的电位中和能力。因此,











































 京公网安备 11010802027423号
京公网安备 11010802027423号