当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and pharmacological evaluation of enantiomerically pure endo-configured KOR agonists with 2-azabicyclo[3.2.1]octane scaffold
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1ob01498f Hendrik Jonas 1, 2 , Daniele Aiello 2 , Bastian Frehland 1 , Kirstin Lehmkuhl 1 , Dirk Schepmann 1 , Jens Köhler 1 , Patrizia Diana 2 , Bernhard Wünsch 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2021-09-09 , DOI: 10.1039/d1ob01498f Hendrik Jonas 1, 2 , Daniele Aiello 2 , Bastian Frehland 1 , Kirstin Lehmkuhl 1 , Dirk Schepmann 1 , Jens Köhler 1 , Patrizia Diana 2 , Bernhard Wünsch 1
Affiliation
|
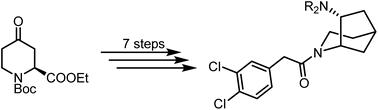
Conformationally restricted bicyclic KOR agonists 10 with an endo-configured amino moiety were synthesized to analyze the bioactive conformation of conformationally flexible KOR agonists such as 2–5. A seven-step synthesis starting with (S)-configured 4-oxopiperidine-2-carboxylate 13 was developed. cis- and trans-configured diesters 12 were obtained in a 3 : 1 ratio via hydrogenation of the α,β-unsaturated ester 14. After establishment of the bicyclic scaffold, a diastereoselective reductive amination of ketone 11 provided exclusively the endo-configured bicyclic amines 10a,b. The 3 : 1 mixtures of enantiomers were separated by chiral HPLC, respectively, leading to enantiomerically pure KOR agonists (1S,5S,7R)-10a,b and (1R,5R,7S)-10a,b (ent-10a,b). The KOR affinity was determined in receptor binding studies with the radioligand [3H]U-69 593. The high KOR affinity of endo-configured amines 10a (Ki = 7 nM) and 10b (Ki = 13 nM) indicates that the dihedral angle of the KOR pharmacophoric element N(pyrrolidine)–C–C–N(phenylacetyl) of 42° is close to the bioactive conformation of more flexible KOR agonists. It should be noted that changing the configuration of potent and selective KOR agonists 10a and 10b led to potent and selective σ1 ligands (e.g. ent-10a Ki(σ1) = 10 nM).
中文翻译:

具有2-氮杂双环[3.2.1]辛烷支架的对映体纯内构型KOR激动剂的合成和药理学评价
合成了具有内构型氨基部分的构象限制性双环 KOR 激动剂10 ,以分析构象灵活的 KOR 激动剂(例如2-5 )的生物活性构象。开发了从 ( S )-构型 4-氧代哌啶-2-羧酸酯13开始的七步合成方法。通过α,β-不饱和酯14的氢化,以3:1的比例获得顺式和反式构型的二酯12 。建立双环支架后,酮11的非对映选择性还原胺化仅提供内构型双环胺10a,b 。分别通过手性 HPLC 分离对映体的 3:1 混合物,得到对映体纯的 KOR 激动剂 (1 S ,5 S ,7 R )- 10a,b和 (1 R ,5 R ,7 S )- 10a,b ( ent - 10a,b )。 KOR 亲和力是在放射性配体 [ 3 H]U-69 593 的受体结合研究中确定的。内构胺10a ( K = 7 nM) 和10b ( K = 13 nM) 的高 KOR 亲和力表明二面角KOR药效团元素N(吡咯烷)–C–C–N(苯乙酰基)的42°的构象接近于更灵活的KOR激动剂的生物活性构象。 应当注意,改变有效且选择性的KOR激动剂10a和10b的构型产生有效且选择性的σ 1配体(例如ent - 10a K ( σ 1 )=10nM)。
更新日期:2021-09-16
中文翻译:

具有2-氮杂双环[3.2.1]辛烷支架的对映体纯内构型KOR激动剂的合成和药理学评价
合成了具有内构型氨基部分的构象限制性双环 KOR 激动剂10 ,以分析构象灵活的 KOR 激动剂(例如2-5 )的生物活性构象。开发了从 ( S )-构型 4-氧代哌啶-2-羧酸酯13开始的七步合成方法。通过α,β-不饱和酯14的氢化,以3:1的比例获得顺式和反式构型的二酯12 。建立双环支架后,酮11的非对映选择性还原胺化仅提供内构型双环胺10a,b 。分别通过手性 HPLC 分离对映体的 3:1 混合物,得到对映体纯的 KOR 激动剂 (1 S ,5 S ,7 R )- 10a,b和 (1 R ,5 R ,7 S )- 10a,b ( ent - 10a,b )。 KOR 亲和力是在放射性配体 [ 3 H]U-69 593 的受体结合研究中确定的。内构胺10a ( K = 7 nM) 和10b ( K = 13 nM) 的高 KOR 亲和力表明二面角KOR药效团元素N(吡咯烷)–C–C–N(苯乙酰基)的42°的构象接近于更灵活的KOR激动剂的生物活性构象。 应当注意,改变有效且选择性的KOR激动剂10a和10b的构型产生有效且选择性的σ 1配体(例如ent - 10a K ( σ 1 )=10nM)。










































 京公网安备 11010802027423号
京公网安备 11010802027423号