European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.ejmech.2021.113848 Xujie Zhang 1 , Lin Sun 1 , Megan E Meuser 2 , Waleed A Zalloum 3 , Shujing Xu 1 , Tianguang Huang 1 , Srinivasulu Cherukupalli 1 , Xiangyi Jiang 1 , Xiao Ding 1 , Yucen Tao 1 , Dongwei Kang 1 , Erik De Clercq 4 , Christophe Pannecouque 4 , Alexej Dick 2 , Simon Cocklin 2 , Xinyong Liu 1 , Peng Zhan 1
|
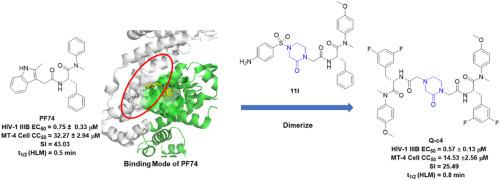
HIV-1 capsid (CA) plays indispensable and multiple roles in the life cycle of HIV-1, become an attractive target in antiviral therapy. Herein, we report the design, synthesis, and mechanism study of a novel series of dimerized phenylalanine derivatives as HIV-1 capsid inhibitors using 2-piperazineone or 2,5-piperazinedione as a linker. The structure-activity relationship (SAR) indicated that dimerized phenylalanines were more potent than monomers of the same chemotype. Further, the inclusion of fluorine substituted phenylalanine and methoxyl substituted aniline was found to be beneficial for antiviral activity. From the synthesized series, Q-c4 was found to be the most potent compound with an EC50 value of 0.57 μM, comparable to PF74. Interestingly, Q-c4 demonstrated a slightly higher affinity to the CA monomer than the CA hexamer, commensurate with its more significant effect in the late-stage of the HIV-1 lifecycle. Competitive SPR experiments with peptides from CPSF6 and NUP153 revealed that Q-c4 binds to the interprotomer pocket of hexameric CA as designed. Single-round infection assays showed that Q-c4 interferes with the HIV-1 life cycle in a dual-stage manner, affecting both pre-and post-integration. Stability assays in human plasma and human liver microsomes indicated that although Q-c4 has improved stability over PF74, this kind of inhibitor still requires further optimization. And the results of the online molinspiration software predicted that Q-c4 has desirable physicochemical properties but some properties still have some violation from the Lipinski rule of five. Overall, the dimerized phenylalanines are promising novel platforms for developing future HIV-1 CA inhibitors with considerable potential for optimization.
中文翻译:

二聚苯丙氨酸衍生物作为新型 HIV-1 衣壳抑制剂的设计、合成和机制研究
HIV-1衣壳(CA)在HIV-1的生命周期中扮演着不可或缺的多重角色,成为抗病毒治疗的一个有吸引力的靶点。在此,我们报告了使用 2-哌嗪酮或 2,5-哌嗪二酮作为接头的新型二聚苯丙氨酸衍生物系列作为 HIV-1 衣壳抑制剂的设计、合成和机制研究。构效关系 (SAR) 表明二聚苯丙氨酸比相同化学型的单体更有效。此外,发现包含氟取代的苯丙氨酸和甲氧基取代的苯胺对抗病毒活性有益。在合成的系列中,发现Q-c4是最有效的化合物,其 EC 50值为 0.57 μM,与PF74相当。有趣的是,Q-c4对 CA 单体的亲和力略高于 CA 六聚体,与其在 HIV-1 生命周期后期的更显着影响相称。使用来自 CPSF6 和 NUP153 的肽进行的竞争性 SPR 实验表明,Q-c4按照设计与六聚体 CA 的原体间口袋结合。单轮感染测定表明,Q-c4以双阶段方式干扰 HIV-1 生命周期,影响整合前和整合后。人血浆和人肝微粒体的稳定性测定表明,虽然Q-c4的稳定性优于PF74,但这种抑制剂仍需要进一步优化。而在线 molinspiration 软件的结果预测,Q-c4具有理想的物理化学性质,但某些性质仍然违反了 Lipinski 的五法则。总体而言,二聚苯丙氨酸是开发未来 HIV-1 CA 抑制剂的有前途的新平台,具有相当大的优化潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号