Water Research ( IF 11.4 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.watres.2021.117669 Tao Yang 1 , Jiamin Mai 1 , Sisi Wu 1 , Weikang Luo 1 , Mengyang Zhu 1 , Ping Liang 2 , Lin Guo 1 , Jing Chen 1 , Jianbo Jia 1 , Jun Ma 3
|
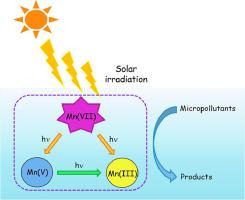
Herein, permanganate [Mn(VII)] was activated by simulated solar (SS) (SS/Mn(VII)), resulting in rapid degradation of micropollutants in several minutes, with rates of target micropollutants outnumbered those in the Mn(VII) alone and SS. To explore the mechanism in this process, 4-cholorphenol (4-CP), p-hydroxybenzoic acid (p-HBA), and enrofloxacin (ENR) were selected as model compounds. Lines of evidence indicated that reactive manganese species (RMnS) (i.e., Mn(III) and Mn(V)) rather than radicals from Mn(VII) photolysis participated in the conversion of model compounds. Interestingly, roles of RMnS differed among three model compounds, suggesting their selectivity toward micropollutants. Increasing Mn(VII) dosage proved greater micropollutant degradation, while impacts of pH on SS/Mn(VII) performance varied among model compounds. P-HBA and ENR showed the lowest degradation efficiency at alkaline, whereas 4-CP demonstrated the best performance at alkaline, indicating the reactivity of RMnS varied toward micropollutants at different pH values. The quantum yield of Mn(VII) was 8.36 ± 0.03 X 10−6 mol Einstein−1 at pH 7.0. Effects of common co-existing constituents (Cl−, HCO3−, and humic acid (HA)) on micropollutant degradation by SS/Mn(VII) were examined. Specifically, HCO3− positively influenced the 4-CP and p-HBA degradation, whereas ENR was not affected, likely owing to the selectivity of RMnS-HCO3− complexes. HA was conducive to degrade p-HBA due to the production of RMnS-HA complexes, but unfavorable for ENR and 4-CP degradation because of the competitive light absorption and Mn(VII). Furthermore, a number of degradation products of 4-CP, p-HBA, and ENR were identified and possible pathways were proposed accordingly. The effectiveness of this process for micropollutant degradation in real waters, natural sunlight, ultraviolet and visible light via cut-off filtering SS emission was confirmed. This work revealed a great potential of applying SS/Mn(VII) for the marked degradation of micropollutants and facilitated the understandings of Mn(III)/Mn(V) behaviors.
中文翻译:

深入了解模拟太阳辐射下高锰酸盐的增强活化:锰物种的快速形成
在此,高锰酸盐 [Mn(VII)] 被模拟太阳能 (SS) (SS/Mn(VII)) 激活,导致微污染物在几分钟内快速降解,目标微污染物的数量超过了单独的 Mn(VII)和SS。为了探究这一过程的机理,4-氯苯酚(4-CP)、对羟基苯甲酸(p-HBA) 和恩诺沙星 (ENR) 作为模型化合物。一系列证据表明,活性锰物质 (RMnS)(即 Mn(III) 和 Mn(V))而不是来自 Mn(VII) 光解的自由基参与了模型化合物的转化。有趣的是,RMnS 在三种模型化合物中的作用不同,表明它们对微污染物具有选择性。增加 Mn(VII) 剂量证明微污染物降解更大,而 pH 值对 SS/Mn(VII) 性能的影响因模型化合物而异。P- HBA 和 ENR 在碱性条件下表现出最低的降解效率,而 4-CP 在碱性条件下表现出最好的性能,表明 RMnS 在不同 pH 值下对微污染物的反应性不同。Mn(VII) 的量子产率为 8.36 ± 0.03 X 10 -6 摩尔爱因斯坦-1在 pH 7.0。检查了常见的共存成分(Cl -、HCO 3 -和腐殖酸 (HA))对 SS/Mn(VII) 对微污染物降解的影响。具体而言,HCO 3 -对4-CP 和p -HBA 降解产生积极影响,而ENR 没有受到影响,这可能是由于RMnS-HCO 3 -复合物的选择性。由于产生 RMnS-HA 复合物,HA 有利于降解p -HBA,但由于竞争性光吸收和 Mn(VII),不利于 ENR 和 4-CP 的降解。此外,许多 4-CP、p的降解产物-HBA 和 ENR 被确定并相应地提出了可能的途径。证实了该过程通过截止过滤 SS 发射在实际水域、自然阳光、紫外线和可见光中降解微污染物的有效性。这项工作揭示了应用 SS/Mn(VII) 显着降解微污染物的巨大潜力,并促进了对 Mn(III)/Mn(V) 行为的理解。










































 京公网安备 11010802027423号
京公网安备 11010802027423号