Biochemical and Biophysical Research Communications ( IF 3.1 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.bbrc.2021.09.016 Rachel M Johnson 1 , Xin Zhang 1 , Sarah J Piper 1 , Theodore J Nettleton 2 , Teresa H Vandekolk 2 , Christopher J Langmead 1 , Radostin Danev 3 , Patrick M Sexton 1 , Denise Wootten 1
|
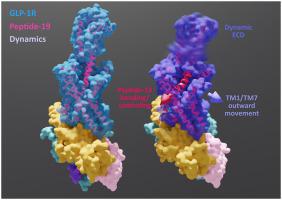
Dual agonists that can activate both the glucagon-like peptide-1 receptor (GLP-1R) and the gastric inhibitory polypeptide receptor (GIPR) have demonstrated high efficacy for the treatment of metabolic disease. Peptide-19 is a prototypical dual agonist that has high potency at both GLP-1R and GIPR but has a distinct signalling profile relative to the native peptides at the cognate receptors. In this study, we solved the structure of peptide-19 bound to the GLP-1R in complex with Gs protein, and compared the structure and dynamics of this complex to that of published structures of GLP-1R:Gs in complex with other receptor agonists. Unlike other peptide-bound receptor complexes, peptide-19:GLP-1R:Gs demonstrated a more open binding pocket where transmembrane domain (TM) 6, TM7 and the interconnecting extracellular loop 3 (ECL3) were located away from the peptide, with no interactions between peptide-19 and TM6/ECL3. Analysis of conformational variance of the complex revealed that peptide-19 was highly dynamic and underwent binding and unbinding motions facilitated by the more open TM binding pocket. Both the consensus structure of the GLP-1R complex with peptide-19 and the dynamics of this complex were distinct from previously described GLP-1R structures providing unique insights into the mode of GLP-1R activation by this dual agonist.
中文翻译:

双肠促胰岛素受体激动剂肽 19 的冷冻电镜结构,与胰高血糖素样肽 1 受体复合
可以激活胰高血糖素样肽-1受体(GLP-1R)和胃抑制多肽受体(GIPR)的双重激动剂已证明对代谢疾病的治疗具有很高的疗效。Peptide-19 是一种典型的双重激动剂,对 GLP-1R 和 GIPR 都具有高效力,但相对于同源受体的天然肽而言,具有不同的信号传导特征。在这项研究中,我们解决了与 GLP-1R 结合的肽 19 与 Gs 蛋白复合物的结构,并将该复合物的结构和动力学与已发表的 GLP-1R:Gs 与其他受体激动剂复合物的结构和动力学进行了比较. 与其他肽结合受体复合物不同,肽 19:GLP-1R:Gs 表现出更开放的结合口袋,其中跨膜结构域 (TM) 6、TM7 和相互连接的细胞外环 3 (ECL3) 远离肽,肽 19 和 TM6/ECL3 之间没有相互作用。复合物的构象变化分析表明,肽 19 是高度动态的,并且经历了由更开放的 TM 结合口袋促进的结合和解除结合运动。具有肽 19 的 GLP-1R 复合物的共有结构和该复合物的动力学都不同于先前描述的 GLP-1R 结构,从而为这种双重激动剂的 GLP-1R 激活模式提供了独特的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号