Applied Surface Science ( IF 6.3 ) Pub Date : 2021-09-16 , DOI: 10.1016/j.apsusc.2021.151240 Grêce Abdallah 1, 2 , Rim Bitar 2 , Savita Kaliya Perumal Veerapandian 2 , Jean-Marc Giraudon 1 , Nathalie De Geyter 2 , Rino Morent 2 , Jean-François Lamonier 1
|
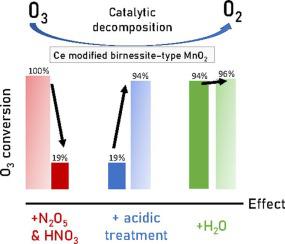
In this study, HNO3 treated Ce modified birnessite–type MnO2 (CexMn-AT; x = 0.01,0.1,0.2,0.5) have been designed for decomposition of ozone at low temperature (20–40 °C) in the absence or presence of nitrogen containing co-pollutants and water. The best catalyst Ce0.01Mn-AT exhibits stable ozone conversion of 94 % in nearly dry air (300 ppm of ozone, RH = 0.7 %, GHSV = 1200 L/(g.h), 20 °C) in the presence of N2O5/HNO3 pollutants. However, the ozone conversion drops to 64 % for the undoped catalyst showing the beneficial role of cerium. Additionally, the Ce0.1Mn-AT catalyst shows a stable ozone conversion of 91 % after 5 h on stream in moist air (RH = 30 %, 30 °C) in the same operating conditions as before. The high tolerance of the best acid-treated catalysts to co-pollutants and water can be explained by the high density of acid sites and oxygen vacancies which facilitate the adsorption and decomposition of ozone and allow to minimize the amount of nitrogen containing adspecies which can affect the catalytic performances for ozone decomposition.
中文翻译:

用于低温臭氧分解的酸处理 Ce 改性水钠锰矿型 MnO2:含氮共污染物和水的影响
在这项研究中,HNO 3处理过的 Ce 改性水钠锰矿型 MnO 2 (Ce x Mn-AT; x = 0.01,0.1,0.2,0.5) 被设计用于在低温 (20–40 °C) 下分解臭氧。不存在或存在含氮的共同污染物和水。最好的催化剂 Ce 0.01 Mn-AT 在几乎干燥的空气中(300 ppm 臭氧,RH = 0.7 %,GHSV = 1200 L/(gh),20 °C)在 N 2 O存在下表现出稳定的臭氧转化率 94% 5 /HNO 3污染物。然而,未掺杂催化剂的臭氧转化率下降到 64%,显示出铈的有益作用。此外,Ce 0.1在与以前相同的操作条件下,Mn-AT 催化剂在潮湿空气(RH = 30 %,30 °C)中运行 5 小时后显示稳定的臭氧转化率为 91%。最好的酸处理催化剂对共污染物和水的高耐受性可以通过高密度的酸位和氧空位来解释,这有助于臭氧的吸附和分解,并允许最大限度地减少可能影响臭氧的含氮物质的数量臭氧分解的催化性能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号