当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rate-Perturbing Single Amino Acid Mutation for Hydrolases: A Statistical Profiling
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jpcb.1c05901 Bailu Yan 1, 2 , Xinchun Ran 1 , Yaoyukun Jiang 1 , Sarah K Torrence 3 , Li Yuan 3 , Qianzhen Shao 1 , Zhongyue J Yang 1, 3, 4, 5
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jpcb.1c05901 Bailu Yan 1, 2 , Xinchun Ran 1 , Yaoyukun Jiang 1 , Sarah K Torrence 3 , Li Yuan 3 , Qianzhen Shao 1 , Zhongyue J Yang 1, 3, 4, 5
Affiliation
|
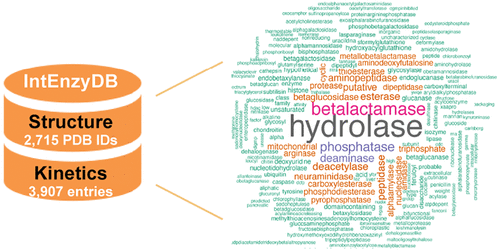
Hydrolases are a critical component for modern chemical, pharmaceutical, and environmental sciences. Identifying mutations that enhance catalytic efficiency presents a roadblock to design and to discover new hydrolases for broad academic and industrial uses. Here, we report the statistical profiling for rate-perturbing mutant hydrolases with a single amino acid substitution. We constructed an integrated structure−kinetics database for hydrolases, IntEnzyDB, which contains 3907 kcats, 4175 KMs, and 2715 Protein Data Bank IDs. IntEnzyDB adopts a relational architecture with a flattened data structure, enabling facile and efficient access to clean and tabulated data for machine learning uses. We conducted statistical analyses on how single amino acids mutations influence the turnover number (i.e., kcat) and efficiency (i.e., kcat/KM), with a particular emphasis on profiling the features for rate-enhancing mutations. The results show that mutation to bulky nonpolar residues with a hydrocarbon chain involves a higher likelihood for rate acceleration than to other types of residues. Linear regression models reveal geometric descriptors of substrate and mutation residues that mediate rate-perturbing outcomes for hydrolases with bulky nonpolar mutations. On the basis of the analyses of the structure−kinetics relationship, we observe that the propensity for rate enhancement is independent of protein sizes. In addition, we observe that distal mutations (i.e., >10 Å from the active site) in hydrolases are significantly more prone to induce efficiency neutrality and avoid efficiency deletion but involve similar propensity for rate enhancement. The studies reveal the statistical features for identifying rate-enhancing mutations in hydrolases, which will potentially guide hydrolase discovery in biocatalysis.
中文翻译:

水解酶的速率扰动单氨基酸突变:统计分析
水解酶是现代化学、制药和环境科学的重要组成部分。识别提高催化效率的突变为设计和发现用于广泛学术和工业用途的新水解酶提供了障碍。在这里,我们报告了具有单个氨基酸替换的速率扰动突变水解酶的统计分析。我们为水解酶构建了一个集成的结构动力学数据库,IntEnzyDB,其中包含 3907 k cat s,4175 K Ms 和 2715 个蛋白质数据库 ID。IntEnzyDB 采用具有扁平化数据结构的关系架构,能够轻松高效地访问用于机器学习的干净和表格数据。我们对单个氨基酸突变如何影响周转数(即k cat)和效率(即k cat / K M),特别强调分析速率增强突变的特征。结果表明,与其他类型的残基相比,突变为具有烃链的庞大非极性残基具有更高的速率加速可能性。线性回归模型揭示了底物和突变残基的几何描述符,它们介导了具有大量非极性突变的水解酶的速率扰动结果。基于对结构动力学关系的分析,我们观察到速率增强的倾向与蛋白质大小无关。此外,我们观察到水解酶中的远端突变(即距活性位点 >10 Å)明显更容易诱导效率中性并避免效率缺失,但涉及类似的速率增强倾向。
更新日期:2021-09-30
中文翻译:

水解酶的速率扰动单氨基酸突变:统计分析
水解酶是现代化学、制药和环境科学的重要组成部分。识别提高催化效率的突变为设计和发现用于广泛学术和工业用途的新水解酶提供了障碍。在这里,我们报告了具有单个氨基酸替换的速率扰动突变水解酶的统计分析。我们为水解酶构建了一个集成的结构动力学数据库,IntEnzyDB,其中包含 3907 k cat s,4175 K Ms 和 2715 个蛋白质数据库 ID。IntEnzyDB 采用具有扁平化数据结构的关系架构,能够轻松高效地访问用于机器学习的干净和表格数据。我们对单个氨基酸突变如何影响周转数(即k cat)和效率(即k cat / K M),特别强调分析速率增强突变的特征。结果表明,与其他类型的残基相比,突变为具有烃链的庞大非极性残基具有更高的速率加速可能性。线性回归模型揭示了底物和突变残基的几何描述符,它们介导了具有大量非极性突变的水解酶的速率扰动结果。基于对结构动力学关系的分析,我们观察到速率增强的倾向与蛋白质大小无关。此外,我们观察到水解酶中的远端突变(即距活性位点 >10 Å)明显更容易诱导效率中性并避免效率缺失,但涉及类似的速率增强倾向。











































 京公网安备 11010802027423号
京公网安备 11010802027423号