当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tracking Ultrafast Fluorescence Switch-On and Color-Tuned Dynamics in Acceptor–Donor–Acceptor Chromophore
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jpcb.1c05936 Wenqi Xu 1, 2 , Lei Wei 1 , Zhengxin Wang 1, 2 , Ruixue Zhu 1 , Jiaming Jiang 1 , Huiyan Liu 1 , Juan Du 3 , Tsu-Chien Weng 1 , Yue-Biao Zhang 1 , Yifan Huang 1 , Weimin Liu 1, 2
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jpcb.1c05936 Wenqi Xu 1, 2 , Lei Wei 1 , Zhengxin Wang 1, 2 , Ruixue Zhu 1 , Jiaming Jiang 1 , Huiyan Liu 1 , Juan Du 3 , Tsu-Chien Weng 1 , Yue-Biao Zhang 1 , Yifan Huang 1 , Weimin Liu 1, 2
Affiliation
|
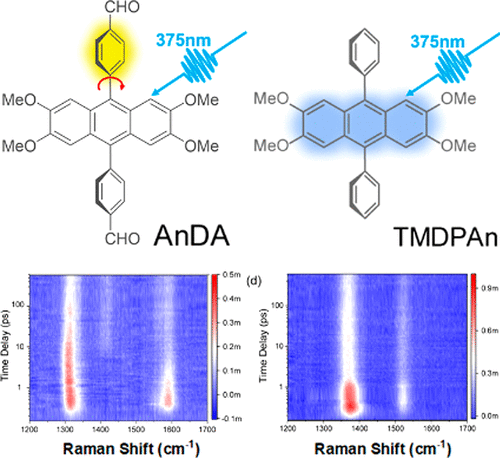
Understanding how the conformational change of conjugated molecules with acceptor–donor–acceptor (A–D–A) architecture affects their physical and optoelectronic properties is critical for determining their ultimate performance in organic electronic devices. Here, we utilized femtosecond transient absorption, time-resolved upconversion photoluminescence spectroscopy, and tunable femtosecond-stimulated Raman spectroscopy, aided by quantum chemical calculations, to systematically investigate the excited state structural dynamics of the intramolecular charge transfer of the tetramethoxy anthracene-based fluorophore 2,3,6,7-tetramethoxy 9,10-dibenzaldehydeanthracene (AnDA) and its derivative 2,3,6,7-tetramethoxy 9,10-diphenylanthracene (TMDPAn) in chloroform. In the AnDA molecule, the tetramethoxy anthracene and benzaldehyde moieties exhibit a strong ability to donate and withdraw electrons. Upon photoexcitation, AnDA shows intriguing ultrafast fluorescence switch-on and red shift dynamics on charge transfer states, and the temporal evolution of AnDA recorded by ultrafast spectroscopy reveals a dynamic picture of two-step intramolecular charge transfer assisted by ultrafast conformational changes and solvation processes. Removing the aldehyde group from TMDPAn significantly decreases the electron pulling capacity of the phenyl unit and disables charge transfer characteristics.
中文翻译:

跟踪受体-供体-受体发色团中的超快荧光开启和颜色调谐动力学
了解具有受体-供体-受体(A-D-A)结构的共轭分子的构象变化如何影响它们的物理和光电特性对于确定它们在有机电子器件中的最终性能至关重要。在这里,我们利用飞秒瞬态吸收、时间分辨上转换光致发光光谱和可调飞秒受激拉曼光谱,在量子化学计算的帮助下,系统地研究了四甲氧基蒽基荧光团 2 分子内电荷转移的激发态结构动力学,3,6,7-四甲氧基 9,10-二苯甲醛蒽 (AnDA) 及其衍生物 2,3,6,7-四甲氧基 9,10-二苯基蒽 (TMDPAn) 的氯仿溶液。在 AnDA 分子中,四甲氧基蒽和苯甲醛部分表现出很强的给电子和取电子能力。在光激发下,AnDA 在电荷转移状态上显示出有趣的超快荧光开启和红移动力学,超快光谱记录的 AnDA 的时间演变揭示了由超快构象变化和溶剂化过程辅助的两步分子内电荷转移的动态图像。从 TMDPAn 中去除醛基会显着降低苯基单元的电子拉力能力,并使电荷转移特性失效。超快光谱记录的 AnDA 的时间演变揭示了由超快构象变化和溶剂化过程辅助的两步分子内电荷转移的动态图。从 TMDPAn 中去除醛基会显着降低苯基单元的电子拉力能力,并使电荷转移特性失效。超快光谱记录的 AnDA 的时间演变揭示了由超快构象变化和溶剂化过程辅助的两步分子内电荷转移的动态图像。从 TMDPAn 中去除醛基会显着降低苯基单元的电子拉力能力,并使电荷转移特性失效。
更新日期:2021-09-30
中文翻译:

跟踪受体-供体-受体发色团中的超快荧光开启和颜色调谐动力学
了解具有受体-供体-受体(A-D-A)结构的共轭分子的构象变化如何影响它们的物理和光电特性对于确定它们在有机电子器件中的最终性能至关重要。在这里,我们利用飞秒瞬态吸收、时间分辨上转换光致发光光谱和可调飞秒受激拉曼光谱,在量子化学计算的帮助下,系统地研究了四甲氧基蒽基荧光团 2 分子内电荷转移的激发态结构动力学,3,6,7-四甲氧基 9,10-二苯甲醛蒽 (AnDA) 及其衍生物 2,3,6,7-四甲氧基 9,10-二苯基蒽 (TMDPAn) 的氯仿溶液。在 AnDA 分子中,四甲氧基蒽和苯甲醛部分表现出很强的给电子和取电子能力。在光激发下,AnDA 在电荷转移状态上显示出有趣的超快荧光开启和红移动力学,超快光谱记录的 AnDA 的时间演变揭示了由超快构象变化和溶剂化过程辅助的两步分子内电荷转移的动态图像。从 TMDPAn 中去除醛基会显着降低苯基单元的电子拉力能力,并使电荷转移特性失效。超快光谱记录的 AnDA 的时间演变揭示了由超快构象变化和溶剂化过程辅助的两步分子内电荷转移的动态图。从 TMDPAn 中去除醛基会显着降低苯基单元的电子拉力能力,并使电荷转移特性失效。超快光谱记录的 AnDA 的时间演变揭示了由超快构象变化和溶剂化过程辅助的两步分子内电荷转移的动态图像。从 TMDPAn 中去除醛基会显着降低苯基单元的电子拉力能力,并使电荷转移特性失效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号