当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dynamics of Reaction CH3CHI + O2 Investigated via Infrared Emission of Products CO, CO2, and OH
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jpca.1c05610 Ya-Tsang Ji, Yuan-Pern Lee
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jpca.1c05610 Ya-Tsang Ji, Yuan-Pern Lee
|
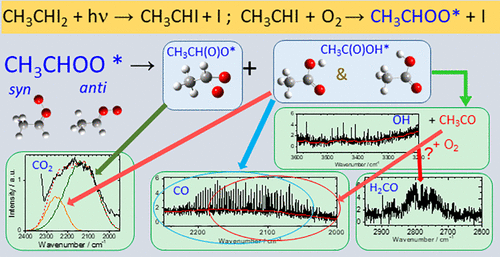
The reaction CH3CHI + O2 has been commonly employed in laboratories to produce a methyl-substituted Criegee intermediate CH3CHOO, but the detailed dynamics of this reaction remain unexplored. We carried out this reaction by irradiating a flowing mixture of CH3CHI2 (∼70 mTorr) and O2 (∼4 and 8 Torr) at 308 or 248 nm and observed infrared emission of the products with a step-scan Fourier-transform spectrometer. Upon irradiation at 248 nm with O2 ∼4 Torr, a Boltzmann distribution of CO (v ≤ 4, J ≤ 25) with average vibrational energy (12 ± 2) kJ mol–1 and of OH (v = 1, J ≤ 5.5) were observed and assigned to be produced from the decomposition of CH3C(O)OH* to form CO + CH3OH and OH + CH3CO, respectively. The observed broadband emission of CO2 was simulated with two vibrational distributions of average energies (42 ± 3) and (114 ± 6) kJ mol–1 and assigned to be produced from the decomposition of CH3C(O)OH* and (methyl dioxirane)*, respectively. The results upon irradiation of the sample at 308 nm are similar, likely indicating a small fraction of energy partition into these products and rapid thermalization of CH3CHI*. Compared with reaction CH2I + O2, the title reaction yielded products with much less internal excitation, consistent with the expectation that these observed products receive much less fraction of available energy upon fragmentation when an additional methyl moiety was present in the parent. The large-v component of CO observed in experiments of CH2I + O2 at 248 nm, produced from secondary reaction HCO + O2, was absent in this work because the corresponding secondary reaction CH3CO + O2 in decomposition of CH3CHOO* produces α-lactone + OH or H2CO + CO + OH.
中文翻译:

通过产品 CO、CO2 和 OH 的红外发射研究 CH3CHI + O2 的反应动力学
CH 3 CHI + O 2反应通常在实验室中用于生产甲基取代的 Criegee 中间体 CH 3 CHOO,但该反应的详细动力学仍未得到探索。我们通过在 308 或 248 nm 处照射 CH 3 CHI 2 (~70 mTorr) 和 O 2 (~4 和 8 Torr)的流动混合物来进行该反应,并通过步进扫描傅里叶变换观察产物的红外发射光谱仪。在 248 nm 处用 O 2 ∼4 Torr照射后,CO ( v ≤ 4, J ≤ 25)的玻尔兹曼分布具有平均振动能量 (12 ± 2) kJ mol –1和 OH ( v= 1, J ≤ 5.5) 被观察到并被指定为由 CH 3 C(O)OH*分解生成CO + CH 3 OH 和 OH + CH 3 CO。观察到的 CO 2宽带发射模拟了两种平均能量 (42 ± 3) 和 (114 ± 6) kJ mol –1 的振动分布,并指定为由 CH 3 C(O)OH* 和 (甲基二环氧乙烷)*。在 308 nm 下照射样品后的结果相似,可能表明一小部分能量分配到这些产品中,并且 CH 3 CHI*快速热化。与反应 CH 2 I + O 2 相比,标题反应产生的产物具有更少的内部激发,这与当母体中存在额外的甲基部分时这些观察到的产物在断裂时获得更少的可用能量分数的预期一致。所述large- v CO的组分在CH的实验中观察到2我+ O 2在248nm,从次级反应HCO + O产生2,就是在这样的工作,因为不存在对应的辅助反应CH 3 CO + O 2在CH的分解3 CHOO* 生成 α-内酯 + OH 或 H 2 CO + CO + OH。
更新日期:2021-09-30
中文翻译:

通过产品 CO、CO2 和 OH 的红外发射研究 CH3CHI + O2 的反应动力学
CH 3 CHI + O 2反应通常在实验室中用于生产甲基取代的 Criegee 中间体 CH 3 CHOO,但该反应的详细动力学仍未得到探索。我们通过在 308 或 248 nm 处照射 CH 3 CHI 2 (~70 mTorr) 和 O 2 (~4 和 8 Torr)的流动混合物来进行该反应,并通过步进扫描傅里叶变换观察产物的红外发射光谱仪。在 248 nm 处用 O 2 ∼4 Torr照射后,CO ( v ≤ 4, J ≤ 25)的玻尔兹曼分布具有平均振动能量 (12 ± 2) kJ mol –1和 OH ( v= 1, J ≤ 5.5) 被观察到并被指定为由 CH 3 C(O)OH*分解生成CO + CH 3 OH 和 OH + CH 3 CO。观察到的 CO 2宽带发射模拟了两种平均能量 (42 ± 3) 和 (114 ± 6) kJ mol –1 的振动分布,并指定为由 CH 3 C(O)OH* 和 (甲基二环氧乙烷)*。在 308 nm 下照射样品后的结果相似,可能表明一小部分能量分配到这些产品中,并且 CH 3 CHI*快速热化。与反应 CH 2 I + O 2 相比,标题反应产生的产物具有更少的内部激发,这与当母体中存在额外的甲基部分时这些观察到的产物在断裂时获得更少的可用能量分数的预期一致。所述large- v CO的组分在CH的实验中观察到2我+ O 2在248nm,从次级反应HCO + O产生2,就是在这样的工作,因为不存在对应的辅助反应CH 3 CO + O 2在CH的分解3 CHOO* 生成 α-内酯 + OH 或 H 2 CO + CO + OH。











































 京公网安备 11010802027423号
京公网安备 11010802027423号