当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Study of the N7 Regioselective Glycosylation of 6-Chloropurine and 2,6-Dichloropurine with Tin and Titanium Tetrachloride
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.joc.1c01186 Lenka Tranová 1 , Jakub Stýskala 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.joc.1c01186 Lenka Tranová 1 , Jakub Stýskala 1
Affiliation
|
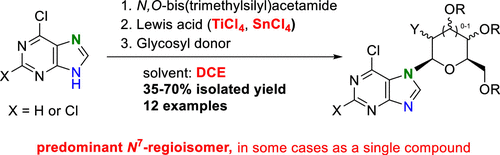
6-Chloropurine and 2,6-dichloropurine were regioselectively glycosylated at position 7 to give the corresponding peracetylated N7-nucleosides, which can be suitable for other purine transformations. In this work, we study the distribution of N7/N9-isomers produced via the Vorbrüggen method under different conditions, using an N-trimethylsilylated purine derivative and SnCl4 or TiCl4 as a catalyst. The main effort is devoted to reversing the disadvantageous predominant selectivity of most glycosylation reactions at the N9 position and thus to determining conditions that maximize the regioselectivity of glycosylation toward the desired N7-isomer.
中文翻译:

6-氯嘌呤和2,6-二氯嘌呤与四氯化锡和四氯化钛的N7区域选择性糖基化研究
6-氯嘌呤和 2,6-二氯嘌呤在 7 位进行区域选择性糖基化,得到相应的全乙酰化N 7 -核苷,可适用于其他嘌呤转化。在这项工作中,我们使用N-三甲基甲硅烷基化嘌呤衍生物和 SnCl 4或 TiCl 4作为催化剂,研究了在不同条件下通过 Vorbrüggen 方法产生的N 7 / N 9 -异构体的分布。主要努力致力于扭转大多数糖基化反应在N 9处不利的主要选择性位置,从而确定使糖基化对所需N 7异构体的区域选择性最大化的条件。
更新日期:2021-10-01
中文翻译:

6-氯嘌呤和2,6-二氯嘌呤与四氯化锡和四氯化钛的N7区域选择性糖基化研究
6-氯嘌呤和 2,6-二氯嘌呤在 7 位进行区域选择性糖基化,得到相应的全乙酰化N 7 -核苷,可适用于其他嘌呤转化。在这项工作中,我们使用N-三甲基甲硅烷基化嘌呤衍生物和 SnCl 4或 TiCl 4作为催化剂,研究了在不同条件下通过 Vorbrüggen 方法产生的N 7 / N 9 -异构体的分布。主要努力致力于扭转大多数糖基化反应在N 9处不利的主要选择性位置,从而确定使糖基化对所需N 7异构体的区域选择性最大化的条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号