当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Anionic, Chelating C(sp3)/NHC ligand from the Combination of an N-heterobicyclic Carbene and Barbituric Heterocycle
Organometallics ( IF 2.5 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.organomet.1c00458 Idir Benaissa 1 , Katarzyna Gajda 1, 2 , Laure Vendier 1 , Noël Lugan 1 , Anna Kajetanowicz 2 , Karol Grela 2 , Véronique Michelet 3 , Vincent César 1 , Stéphanie Bastin 1
Organometallics ( IF 2.5 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.organomet.1c00458 Idir Benaissa 1 , Katarzyna Gajda 1, 2 , Laure Vendier 1 , Noël Lugan 1 , Anna Kajetanowicz 2 , Karol Grela 2 , Véronique Michelet 3 , Vincent César 1 , Stéphanie Bastin 1
Affiliation
|
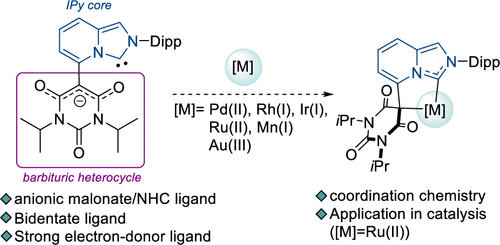
The coordination chemistry of the anionic NHC 1– based on an imidazo[1,5-a]pyridin-3-ylidene (IPy) platform substituted at the C5 position by an anionic barbituric heterocycle was studied with d6 (Ru(II), Mn(I)) and d8 (Pd(II), Rh(I), Ir(I), Au(III)) transition-metal centers. While the anionic barbituric heterocycle is planar in the zwitterionic NHC precursor 1·H, NMR spectroscopic analyses supplemented by X-ray diffraction studies evidenced the chelating behavior of ligand 1– through the carbenic and the malonic carbon atoms in all of the complexes, resulting from a deformation of the lateral barbituric heterocycle. The complexes were obtained by reaction of the free carbene with the appropriate metal precursor, except for the Au(III) complex 10, which was obtained by oxidation of the antecedent gold(I) complex [AuCl(1)]− with PhICl2 as an external oxidant. During the course of the process, the kinetic gold(I) intermediate 9 resulting from the oxidation of the malonic carbon of the barbituric moiety was isolated upon crystallization from the reaction mixture. The νCO stretching frequencies recorded for complex [Rh(1)(CO)2] (5) demonstrated the strong donating character of the malonate-C(sp3)/NHC ligand 1–. The ruthenium complex [Ru(1)Cl(p-cymene)] (11) was implemented as a precatalyst in the dehydrogenative synthesis of carboxylic acid derivatives from primary alcohols and exhibited high activities at low catalyst loadings (25–250 ppm) and a large tolerance toward functional groups.
中文翻译:

来自 N-杂双环卡宾和巴比妥杂环组合的阴离子螯合 C(sp3)/NHC 配体
阴离子NHC 1的配位化学——基于咪唑并[1,5 - a ]吡啶-3-亚基(IPy)平台,在C5位被阴离子巴比妥杂环取代,用d 6(Ru(II), Mn(I)) 和 d 8 (Pd(II)、Rh(I)、Ir(I)、Au(III)) 过渡金属中心。虽然阴离子巴比妥杂环在两性离子 NHC 前体1 ·H 中是平面的,但通过 X 射线衍射研究补充的 NMR 光谱分析证明了配体1的螯合行为-通过所有复合物中的碳原子和丙二酸碳原子,由侧向巴比妥杂环的变形产生。配合物是通过游离卡宾与适当的金属前体反应获得的,除了 Au(III) 配合物10,它是通过氧化前体金 (I) 配合物 [AuCl( 1 )] -与 PhICl 2作为一种外部氧化剂。在该方法过程中,由巴比妥部分的丙二酸碳氧化产生的动力学金(I)中间体9在从反应混合物结晶时分离。为复数 [Rh( 1 )(CO)记录的 ν CO伸缩频率2 ] ( 5 ) 证明了丙二酸-C(sp 3 )/NHC 配体1 –的强捐赠特性。钌配合物 [Ru( 1 )Cl( p- cymene)] ( 11 ) 被用作伯醇脱氢合成羧酸衍生物的预催化剂,并在低催化剂负载量 (25-250 ppm) 和对官能团有很大的耐受性。
更新日期:2021-09-27
中文翻译:

来自 N-杂双环卡宾和巴比妥杂环组合的阴离子螯合 C(sp3)/NHC 配体
阴离子NHC 1的配位化学——基于咪唑并[1,5 - a ]吡啶-3-亚基(IPy)平台,在C5位被阴离子巴比妥杂环取代,用d 6(Ru(II), Mn(I)) 和 d 8 (Pd(II)、Rh(I)、Ir(I)、Au(III)) 过渡金属中心。虽然阴离子巴比妥杂环在两性离子 NHC 前体1 ·H 中是平面的,但通过 X 射线衍射研究补充的 NMR 光谱分析证明了配体1的螯合行为-通过所有复合物中的碳原子和丙二酸碳原子,由侧向巴比妥杂环的变形产生。配合物是通过游离卡宾与适当的金属前体反应获得的,除了 Au(III) 配合物10,它是通过氧化前体金 (I) 配合物 [AuCl( 1 )] -与 PhICl 2作为一种外部氧化剂。在该方法过程中,由巴比妥部分的丙二酸碳氧化产生的动力学金(I)中间体9在从反应混合物结晶时分离。为复数 [Rh( 1 )(CO)记录的 ν CO伸缩频率2 ] ( 5 ) 证明了丙二酸-C(sp 3 )/NHC 配体1 –的强捐赠特性。钌配合物 [Ru( 1 )Cl( p- cymene)] ( 11 ) 被用作伯醇脱氢合成羧酸衍生物的预催化剂,并在低催化剂负载量 (25-250 ppm) 和对官能团有很大的耐受性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号