当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Concerted Multiproton–Multielectron Transfer for the Reduction of O2 to H2O with a Polyoxovanadate Cluster
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-16 , DOI: 10.1021/jacs.1c07076 Alex A Fertig 1 , William W Brennessel 1 , James R McKone 2 , Ellen M Matson 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-09-16 , DOI: 10.1021/jacs.1c07076 Alex A Fertig 1 , William W Brennessel 1 , James R McKone 2 , Ellen M Matson 1
Affiliation
|
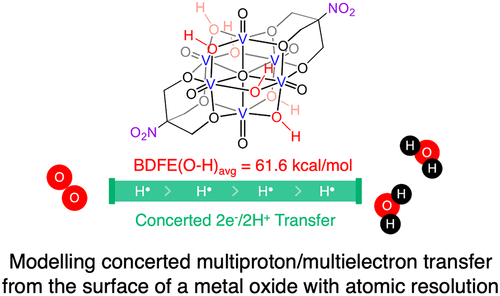
The concerted transfer of protons and electrons enables the activation of small-molecule substrates by bypassing energetically costly intermediates. Here, we present the synthesis and characterization of several hydrogenated forms of an organofunctionalized vanadium oxide assembly, [V6O13(TRIOLNO2)2]2–, and their ability to facilitate the concerted transfer of protons and electrons to O2. Electrochemical analysis reveals that the fully reduced cluster is capable of mediating 2e–/2H+ transfer reactions from surface hydroxide ligands, with an average bond dissociation free energy (BDFE) of 61.6 kcal/mol. Complementary stoichiometric experiments with hydrogen-atom-accepting reagents of established bond strengths confirm that the electrochemically established BDFE predicts the 2H+/2e– transfer reactivity of the assembly. Finally, the reactivity of the reduced polyoxovanadate toward O2 reduction is summarized; our results indicate a stepwise reduction of the substrate, proceeding through H2O2 en route to the formation of H2O. Kinetic isotope effect experiments confirm the participation of hydrogen transfer in the rate-determining step of both the reduction of O2 and H2O2. This work constitutes the first example of hydrogen atom transfer for small-molecule activation with reduced polyoxometalates, where both electron and proton originate from the cluster.
中文翻译:

使用多氧钒酸盐簇将 O2 还原为 H2O 的协同多质子-多电子转移
质子和电子的协同转移能够通过绕过能量昂贵的中间体来激活小分子底物。在这里,我们展示了几种氢化形式的有机功能化氧化钒组件 [V 6 O 13 (TRIOL NO 2 ) 2 ] 2–的合成和表征,以及它们促进质子和电子向 O 2协同转移的能力。电化学分析表明完全还原的簇能够介导 2e – /2H +表面氢氧化物配体的转移反应,平均键解离自由能 (BDFE) 为 61.6 kcal/mol。与已建立键强度的氢原子接受试剂的互补化学计量实验证实,电化学建立的 BDFE 预测了组件的 2H + /2e –转移反应性。最后,总结了还原的多氧钒酸盐对 O 2还原的反应性;我们的结果表明在衬底的逐步减少,至H前进2 ö 2途中H的形成2 O.动力学同位素效应实验确认氢转移的以O二者减少的速率决定步骤的参与2和 H 2 O 2。这项工作构成了使用还原的多金属氧酸盐进行小分子活化的氢原子转移的第一个例子,其中电子和质子都来自簇。
更新日期:2021-09-29
中文翻译:

使用多氧钒酸盐簇将 O2 还原为 H2O 的协同多质子-多电子转移
质子和电子的协同转移能够通过绕过能量昂贵的中间体来激活小分子底物。在这里,我们展示了几种氢化形式的有机功能化氧化钒组件 [V 6 O 13 (TRIOL NO 2 ) 2 ] 2–的合成和表征,以及它们促进质子和电子向 O 2协同转移的能力。电化学分析表明完全还原的簇能够介导 2e – /2H +表面氢氧化物配体的转移反应,平均键解离自由能 (BDFE) 为 61.6 kcal/mol。与已建立键强度的氢原子接受试剂的互补化学计量实验证实,电化学建立的 BDFE 预测了组件的 2H + /2e –转移反应性。最后,总结了还原的多氧钒酸盐对 O 2还原的反应性;我们的结果表明在衬底的逐步减少,至H前进2 ö 2途中H的形成2 O.动力学同位素效应实验确认氢转移的以O二者减少的速率决定步骤的参与2和 H 2 O 2。这项工作构成了使用还原的多金属氧酸盐进行小分子活化的氢原子转移的第一个例子,其中电子和质子都来自簇。










































 京公网安备 11010802027423号
京公网安备 11010802027423号