当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic Properties from Adiabatic and Combustion Calorimetry of Two Polycyclic Aromatic Hydrocarbons, Benz[a]anthracene and Chrysene
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jced.1c00152 Maxim I. Lelet 1 , Vera N. Larina 1 , Elizaveta O. Silyakova 1 , Evgeny V. Suleimanov 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.jced.1c00152 Maxim I. Lelet 1 , Vera N. Larina 1 , Elizaveta O. Silyakova 1 , Evgeny V. Suleimanov 1
Affiliation
|
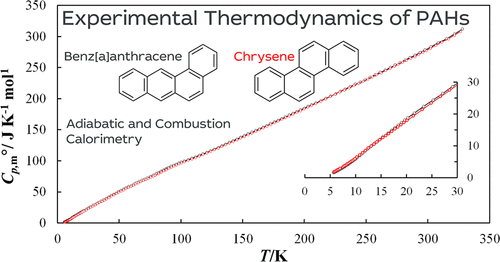
The temperature dependences of the heat capacity of two crystalline polycyclic aromatic hydrocarbons, benz[a]anthracene and chrysene, were investigated using adiabatic calorimetry in the temperature ranges T = 5.5–326.4 and 5.7–328.1 K, respectively. Smoothed heat capacity, Cp, mo(T), values between 0 and 320 K are presented, along with values for the standard thermodynamic functions: the enthalpy, Hmo(T) – Hmo(0), the entropy, Smo(T), and the Gibbs free energy, Gmo(T) – Hmo(0). Using the heat capacity, Cp, mo(T), data, the standard molar third-law entropies at T = 298.15 K, Smo, are calculated to be 272.1 ± 1.6 J·K–1·mol–1 for benz[a]anthracene and 274.1 ± 1.7 J·K–1·mol–1 for chrysene. The standard enthalpies of combustion, ΔcHmo, for benz[a]anthracene and chrysene have been determined using an isothermal combustion calorimeter with a stationary bomb. These new experimental results, together with literature data, are used to calculate the enthalpies of formation, ΔfHmo, and Gibbs free energies of formation, ΔfGmo, giving ΔfHmo(298.15 K, benz[a]anthracene, cr) = 143.3 ± 22.3 kJ · mol–1 and ΔfGmo(298.15 K, benz[a]anthracene, cr) = 326.8 ± 22.4 kJ · mol–1 for benz[a]anthracene and ΔfHmo(298.15 K, chrysene, cr) = 110.7 ± 13.0 kJ · mol–1 and ΔfGmo(298.15 K, chrysene, cr) = 293.6 ± 13.2 kJ · mol–1 for chrysene.
中文翻译:

两种多环芳烃,苯并[a]蒽和屈的绝热和燃烧量热法的热力学性质
分别在T = 5.5-326.4 和 5.7-328.1 K的温度范围内使用绝热量热法研究了两种结晶多环芳烃,苯并 [a] 蒽和屈的热容量的温度依赖性。显示了平滑热容C p、m o ( T )、0 到 320 K 之间的值,以及标准热力学函数的值:焓H m o ( T ) – H m o (0)、熵, S m o ( T ) 和吉布斯自由能G m o( T ) – H m o (0)。使用的热容量,Ç p,米Ô(Ť),数据,在所述标准摩尔第三定律熵T = 298.15 K,小号米ø,被计算为272.1±1.6Ĵ·K -1 ·摩尔-1为苯 [a] 蒽和 274.1 ± 1.7 J·K –1 ·mol –1为 chrysene。标准燃烧焓,Δ c H m o, 对于苯并 [a] 蒽和 chrysene 已使用带有固定炸弹的等温燃烧量热计测定。这些新的实验结果与文献数据一起用于计算生成焓 Δ f H m o和吉布斯生成自由能 Δ f G m o,得到 Δ f H m o (298.15 K, benz[ a]蒽,cr) = 143.3 ± 22.3 kJ · mol –1和 Δ f G m o (298.15 K, 苯 [a] 蒽, cr) = 326.8 ± 22.4 kJ · mol –1苯并 [a] 蒽和 Δ ˚F ħ米ö(298.15 K, chrysene, cr) = 110.7 ± 13.0 kJ · mol –1和 Δ f G m o (298.15 K, chrysene, cr) = 293.6 ± 13.2 kJ · mol –1为 chrysene。
更新日期:2021-10-14
中文翻译:

两种多环芳烃,苯并[a]蒽和屈的绝热和燃烧量热法的热力学性质
分别在T = 5.5-326.4 和 5.7-328.1 K的温度范围内使用绝热量热法研究了两种结晶多环芳烃,苯并 [a] 蒽和屈的热容量的温度依赖性。显示了平滑热容C p、m o ( T )、0 到 320 K 之间的值,以及标准热力学函数的值:焓H m o ( T ) – H m o (0)、熵, S m o ( T ) 和吉布斯自由能G m o( T ) – H m o (0)。使用的热容量,Ç p,米Ô(Ť),数据,在所述标准摩尔第三定律熵T = 298.15 K,小号米ø,被计算为272.1±1.6Ĵ·K -1 ·摩尔-1为苯 [a] 蒽和 274.1 ± 1.7 J·K –1 ·mol –1为 chrysene。标准燃烧焓,Δ c H m o, 对于苯并 [a] 蒽和 chrysene 已使用带有固定炸弹的等温燃烧量热计测定。这些新的实验结果与文献数据一起用于计算生成焓 Δ f H m o和吉布斯生成自由能 Δ f G m o,得到 Δ f H m o (298.15 K, benz[ a]蒽,cr) = 143.3 ± 22.3 kJ · mol –1和 Δ f G m o (298.15 K, 苯 [a] 蒽, cr) = 326.8 ± 22.4 kJ · mol –1苯并 [a] 蒽和 Δ ˚F ħ米ö(298.15 K, chrysene, cr) = 110.7 ± 13.0 kJ · mol –1和 Δ f G m o (298.15 K, chrysene, cr) = 293.6 ± 13.2 kJ · mol –1为 chrysene。











































 京公网安备 11010802027423号
京公网安备 11010802027423号