当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Metal Ions on Aqueous-Phase Decomposition of α-Hydroxyalkyl-Hydroperoxides Derived from Terpene Alcohols
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.est.1c04635 Mingxi Hu 1 , Kenichi Tonokura 1 , Yu Morino 2 , Kei Sato 2 , Shinichi Enami 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-09-16 , DOI: 10.1021/acs.est.1c04635 Mingxi Hu 1 , Kenichi Tonokura 1 , Yu Morino 2 , Kei Sato 2 , Shinichi Enami 2
Affiliation
|
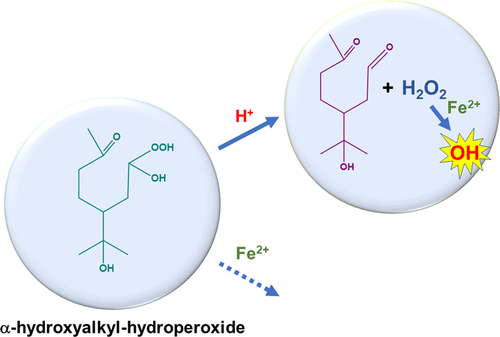
We report the results of a mass spectrometric study of the effects of atmospherically relevant metal ions on the decomposition of α-hydroxyalkyl-hydroperoxides (α-HHs) derived from ozonolysis of α-terpineol in aqueous solutions. By direct mass spectrometric detection of chloride adducts of α-HHs, we assessed the temporal profiles of α-HHs and other products in the presence of metal ions. In addition, reactions between α-HHs and FeCl2 in the presence of excess DMSO showed that the amount of hydroxyl radicals formed in a mixture of α-terpineol, O3, and FeCl2 was 5.7 ± 0.8% of the amount formed in a mixture of H2O2 and FeCl2. The first-order rate constants for the decay of α-HHs produced by ozonolysis of α-terpineol in the presence of 5 mM acetate buffer at a pH of 5.1 ± 0.1 were determined to be (4.5 ± 0.1) × 10–4 s–1 (no metal ions), (4.7 ± 0.2) × 10–4 s–1 (with 0.05 mM Fe2+), (4.7 ± 0.1) × 10–4 s–1 (with 0.05 mM Zn2+), and (4.8 ± 0.2) × 10–4 s–1 (with 0.05 mM Cu2+). We propose that in acidic aqueous media, the reaction of α-HHs with Fe2+ is outcompeted by H+-catalyzed decomposition of α-HHs, which produces the corresponding aldehydes and H2O2, which can in turn react with Fe2+ to form hydroxyl radicals.
中文翻译:

金属离子对萜烯醇衍生α-羟基烷基氢过氧化物水相分解的影响
我们报告了大气相关金属离子对水溶液中α-萜品醇臭氧分解产生的α-羟烷基氢过氧化物(α-HHs)分解影响的质谱研究结果。通过直接质谱检测 α-HHs 的氯化物加合物,我们评估了 α-HHs 和其他产物在金属离子存在下的时间分布。此外,α-HHs 和 FeCl 2在过量 DMSO 存在下的反应表明,α-萜品醇、O 3和 FeCl 2的混合物中形成的羟基自由基的量为 5.7 ± 0.8%。 H 2 O 2和 FeCl 2 的混合物. 在 pH 值为 5.1 ± 0.1 的 5 mM 醋酸盐缓冲液存在下,α-萜品醇臭氧分解产生的 α-HHs 衰减的一级速率常数确定为 (4.5 ± 0.1) × 10 –4 s – 1(无金属离子)、(4.7 ± 0.2) × 10 –4 s –1(含 0.05 mM Fe 2+)、(4.7 ± 0.1)× 10 –4 s –1(含 0.05 mM Zn 2+)和(4.8 ± 0.2) × 10 –4 s –1(含 0.05 mM Cu 2+)。我们建议在酸性水介质中,α-HHs 与 Fe 2+的反应被 H +竞争-催化α-HHs分解,产生相应的醛类和H 2 O 2,H 2 O 2又可以与Fe 2+反应形成羟基自由基。
更新日期:2021-10-06
中文翻译:

金属离子对萜烯醇衍生α-羟基烷基氢过氧化物水相分解的影响
我们报告了大气相关金属离子对水溶液中α-萜品醇臭氧分解产生的α-羟烷基氢过氧化物(α-HHs)分解影响的质谱研究结果。通过直接质谱检测 α-HHs 的氯化物加合物,我们评估了 α-HHs 和其他产物在金属离子存在下的时间分布。此外,α-HHs 和 FeCl 2在过量 DMSO 存在下的反应表明,α-萜品醇、O 3和 FeCl 2的混合物中形成的羟基自由基的量为 5.7 ± 0.8%。 H 2 O 2和 FeCl 2 的混合物. 在 pH 值为 5.1 ± 0.1 的 5 mM 醋酸盐缓冲液存在下,α-萜品醇臭氧分解产生的 α-HHs 衰减的一级速率常数确定为 (4.5 ± 0.1) × 10 –4 s – 1(无金属离子)、(4.7 ± 0.2) × 10 –4 s –1(含 0.05 mM Fe 2+)、(4.7 ± 0.1)× 10 –4 s –1(含 0.05 mM Zn 2+)和(4.8 ± 0.2) × 10 –4 s –1(含 0.05 mM Cu 2+)。我们建议在酸性水介质中,α-HHs 与 Fe 2+的反应被 H +竞争-催化α-HHs分解,产生相应的醛类和H 2 O 2,H 2 O 2又可以与Fe 2+反应形成羟基自由基。











































 京公网安备 11010802027423号
京公网安备 11010802027423号