当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Modeling-Assisted Mechanistic Understanding of the Catalytic Ozonation Process Using Cu–Al Layered Double Hydroxides and Copper Oxide Catalysts
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.est.1c03718 Yuting Yuan 1 , Shikha Garg 1 , Jinxing Ma 1 , T David Waite 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.est.1c03718 Yuting Yuan 1 , Shikha Garg 1 , Jinxing Ma 1 , T David Waite 1
Affiliation
|
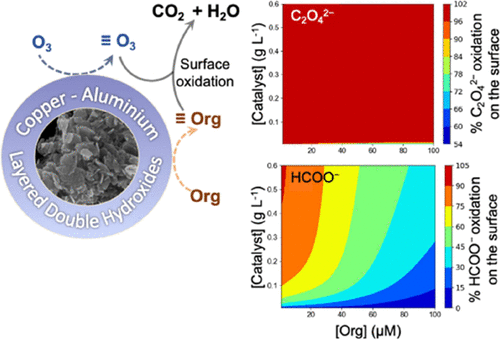
In this study, copper aluminum layered hydroxides (Cu–Al LDHs) and copper oxide (CuO) were utilized as catalysts for heterogeneous catalytic ozonation (HCO). Target compounds oxalate and formate were used with removal by adsorption and oxidation quantified to elucidate the role of the catalyst in contaminant removal. Oxidation of oxalate mostly occurred on the catalyst surface via interaction of surface oxalate complexes with surface-located oxidants. In contrast, the oxidation of formate occurred in the bulk solution as well as on the surface of the catalyst. Measurement of O3 decay kinetics coupled with fluorescence microscopy image analysis corresponding to 7-hydroxycoumarin formation indicates that while surface hydroxyl groups in Cu–Al LDHs facilitate slow decay of O3 resulting in the formation of hydroxyl radicals on the surface, CuO rapidly transforms O3 into surface-located hydroxyl radicals and/or other oxidants. Futile consumption of surface-located oxidants via interaction with the catalyst surface was minimal for Cu–Al-LDHs; however, it becomes significant in the presence of higher CuO dosages. A mechanistic kinetic model has been developed which adequately describes the experimental results obtained and can be used to optimize the process conditions for the application of HCO.
中文翻译:

使用 Cu-Al 层状双氢氧化物和氧化铜催化剂的催化臭氧化过程的动力学建模辅助机理理解
在这项研究中,铜铝层状氢氧化物(Cu-Al LDHs)和氧化铜(CuO)被用作多相催化臭氧化(HCO)的催化剂。目标化合物草酸盐和甲酸盐通过吸附和氧化进行定量去除,以阐明催化剂在污染物去除中的作用。草酸盐的氧化主要通过表面草酸盐配合物与位于表面的氧化剂的相互作用发生在催化剂表面。相反,甲酸盐的氧化发生在本体溶液以及催化剂表面。O 3衰变动力学的测量与对应于 7-羟基香豆素形成的荧光显微镜图像分析表明,虽然 Cu-Al LDH 中的表面羟基促进 O 3 的缓慢衰变导致在表面形成羟基自由基,CuO 迅速将 O 3转化为位于表面的羟基自由基和/或其他氧化剂。对于 Cu-Al-LDHs,表面氧化剂通过与催化剂表面相互作用的无用消耗是最小的;然而,在存在更高的 CuO 剂量时,它变得很重要。已经开发了一个机械动力学模型,该模型充分描述了获得的实验结果,可用于优化应用 HCO 的工艺条件。
更新日期:2021-10-06
中文翻译:

使用 Cu-Al 层状双氢氧化物和氧化铜催化剂的催化臭氧化过程的动力学建模辅助机理理解
在这项研究中,铜铝层状氢氧化物(Cu-Al LDHs)和氧化铜(CuO)被用作多相催化臭氧化(HCO)的催化剂。目标化合物草酸盐和甲酸盐通过吸附和氧化进行定量去除,以阐明催化剂在污染物去除中的作用。草酸盐的氧化主要通过表面草酸盐配合物与位于表面的氧化剂的相互作用发生在催化剂表面。相反,甲酸盐的氧化发生在本体溶液以及催化剂表面。O 3衰变动力学的测量与对应于 7-羟基香豆素形成的荧光显微镜图像分析表明,虽然 Cu-Al LDH 中的表面羟基促进 O 3 的缓慢衰变导致在表面形成羟基自由基,CuO 迅速将 O 3转化为位于表面的羟基自由基和/或其他氧化剂。对于 Cu-Al-LDHs,表面氧化剂通过与催化剂表面相互作用的无用消耗是最小的;然而,在存在更高的 CuO 剂量时,它变得很重要。已经开发了一个机械动力学模型,该模型充分描述了获得的实验结果,可用于优化应用 HCO 的工艺条件。











































 京公网安备 11010802027423号
京公网安备 11010802027423号