当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Accelerating FeIII-Aqua Complex Reduction in an Efficient Solid–Liquid-Interfacial Fenton Reaction over the Mn–CNH Co-catalyst at Near-Neutral pH
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.est.1c04534 Yueshuang Mao 1 , Pengfei Wang 2 , Dongpeng Zhang 1 , Yuguo Xia 3 , Yi Li 4 , Wenlu Zeng 1 , Sihui Zhan 1 , John C Crittenden 5
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-09-15 , DOI: 10.1021/acs.est.1c04534 Yueshuang Mao 1 , Pengfei Wang 2 , Dongpeng Zhang 1 , Yuguo Xia 3 , Yi Li 4 , Wenlu Zeng 1 , Sihui Zhan 1 , John C Crittenden 5
Affiliation
|
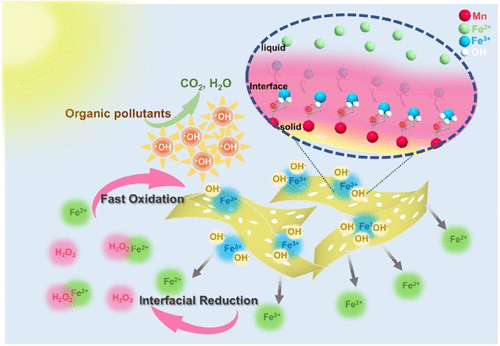
The sluggish regeneration rate of FeII and low operating pH still restrict the wider application of classical Fenton process (FeII/H2O2) for practical water treatment. To overcome these challenges, we exploit the Mn–CNH co-catalyst to construct a solid–liquid interfacial Fenton reaction and accelerate the FeIII/FeII redox cycle at the interface for sustainably generating •OH from H2O2 activation. The Mn–CNH co-catalyst exhibits an excellent regeneration rate of FeII (∼65%) and a high tetracycline removal rate (Kobs) of 0.0541 min–1, which is 19.0 times higher than that of the FeII/H2O2 system (0.0027 min–1) at a near-neutral pH (pH ≈ 5.8), and it also attains 100% degradation of sulfamethoxazole, rhodamine B, and methyl orange. The cyclic mechanism of FeIII/FeII is further elucidated in an atomic scale by combining characterizations and density functional theory calculations, including FeaqIII specific adsorption and the electron-transfer process. Mn active sites can accumulate electrons from the matrix and adsorb FeaqIII to form Mn–Fe bonds at the solid–liquid interface, which accelerate electron transfer from Mn–CNH to FeaqIII and promote the regeneration of FeII at a wide pH range with a lower energy barrier. The regeneration rate of FeII in the Mn–CNH/FeII/H2O2 system outperforms the benchmark Fenton system and other typical metal nanomaterials, which has great potential to be widely applied in actual environment remediation.
中文翻译:

在接近中性 pH 值的 Mn-CNH 助催化剂上,在有效的固-液-界面芬顿反应中加速 FeIII-Aqua 络合物的还原
Fe II缓慢的再生率和较低的操作 pH 值仍然限制了经典 Fenton 工艺(Fe II /H 2 O 2)在实际水处理中的广泛应用。为了克服这些挑战,我们利用 Mn-CNH 助催化剂构建固-液界面芬顿反应并加速界面处的 Fe III /Fe II氧化还原循环,从而可持续地从 H 2 O 2活化中生成• OH 。Mn-CNH 助催化剂表现出优异的 Fe II再生率(~65%) 和0.0541 分钟的高四环素去除率 ( K obs )–1,在接近中性的 pH 值(pH ≈ 5.8)下比 Fe II /H 2 O 2系统(0.0027 min –1)高 19.0 倍,并且它还实现了磺胺甲恶唑、罗丹明 B 的 100% 降解,和甲基橙。通过结合表征和密度泛函理论计算,包括 Fe aq III特异性吸附和电子转移过程,在原子尺度上进一步阐明了 Fe III / Fe II的循环机制。Mn 活性位点可以从基体中积累电子并吸附 Fe aq III在固-液界面形成 Mn-Fe 键,加速电子从 Mn-CNH 转移到 Fe aq III并促进 Fe II在较宽的 pH 范围内以较低的能垒再生。Fe II在Mn-CNH/Fe II /H 2 O 2体系中的再生率优于基准Fenton体系和其他典型的金属纳米材料,在实际环境修复中具有广泛应用的潜力。
更新日期:2021-10-06
中文翻译:

在接近中性 pH 值的 Mn-CNH 助催化剂上,在有效的固-液-界面芬顿反应中加速 FeIII-Aqua 络合物的还原
Fe II缓慢的再生率和较低的操作 pH 值仍然限制了经典 Fenton 工艺(Fe II /H 2 O 2)在实际水处理中的广泛应用。为了克服这些挑战,我们利用 Mn-CNH 助催化剂构建固-液界面芬顿反应并加速界面处的 Fe III /Fe II氧化还原循环,从而可持续地从 H 2 O 2活化中生成• OH 。Mn-CNH 助催化剂表现出优异的 Fe II再生率(~65%) 和0.0541 分钟的高四环素去除率 ( K obs )–1,在接近中性的 pH 值(pH ≈ 5.8)下比 Fe II /H 2 O 2系统(0.0027 min –1)高 19.0 倍,并且它还实现了磺胺甲恶唑、罗丹明 B 的 100% 降解,和甲基橙。通过结合表征和密度泛函理论计算,包括 Fe aq III特异性吸附和电子转移过程,在原子尺度上进一步阐明了 Fe III / Fe II的循环机制。Mn 活性位点可以从基体中积累电子并吸附 Fe aq III在固-液界面形成 Mn-Fe 键,加速电子从 Mn-CNH 转移到 Fe aq III并促进 Fe II在较宽的 pH 范围内以较低的能垒再生。Fe II在Mn-CNH/Fe II /H 2 O 2体系中的再生率优于基准Fenton体系和其他典型的金属纳米材料,在实际环境修复中具有广泛应用的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号