当前位置:
X-MOL 学术
›
Bull. Korean Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into the histidine tautomerism effect on heterodimers of Aβ40
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2021-09-15 , DOI: 10.1002/bkcs.12399 Hao Li 1 , Abbas Salimi 1 , Francis Kirby B. Burnea 2 , Hu Shi 3 , Jin Yong Lee 1
Bulletin of the Korean Chemical Society ( IF 2.3 ) Pub Date : 2021-09-15 , DOI: 10.1002/bkcs.12399 Hao Li 1 , Abbas Salimi 1 , Francis Kirby B. Burnea 2 , Hu Shi 3 , Jin Yong Lee 1
Affiliation
|
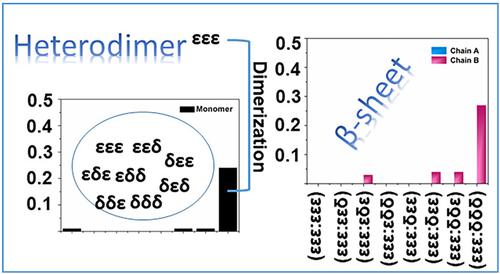
The intrinsic origin of amyloid aggregation has been pursued as a new pathogenesis for Alzheimer's disease (AD). The aggregation mechanisms influenced by histidine tautomerism were previously investigated in Aβ40 and Aβ42 monomers. In the present study, we focus on the structural properties of Aβ40 heterodimer under the influence of histidine. The results of molecular dynamics simulation detected different aggregation trends were detected in different heterodimers formed by the εεε isomer and other isomers. In the (εεε:δδδ) dimer, the highest β-sheet content was obtained in the δδδ chain, which is in agreement with our previous studies that δδδ monomer is the most easily formed β-sheet secondary structure in the monomer. Further analysis confirmed that (εεε:δδδ) dimers more easily aggregate into fibrils in comparison with other heterodimers. This research will help in understanding the tautomeric effect on Aβ heterodimers, thereby helping to figure out the pathogenesis of AD.
中文翻译:

深入了解组氨酸互变异构对 Aβ40 异二聚体的影响
淀粉样蛋白聚集的内在起源已被视为阿尔茨海默病 (AD) 的新发病机制。先前在 Aβ40 和 Aβ42 单体中研究了受组氨酸互变异构影响的聚集机制。在本研究中,我们专注于组氨酸影响下 Aβ40 异源二聚体的结构特性。分子动力学模拟结果检测到εεε异构体和其他异构体形成的不同异二聚体存在不同的聚集趋势。在(εεε:δδδ)二聚体中,δδδ链中β-折叠含量最高,这与我们之前的研究一致,即δδδ单体是单体中最容易形成的β-折叠二级结构。进一步的分析证实,与其他异源二聚体相比,(εεε:δδδ) 二聚体更容易聚集成原纤维。
更新日期:2021-11-22
中文翻译:

深入了解组氨酸互变异构对 Aβ40 异二聚体的影响
淀粉样蛋白聚集的内在起源已被视为阿尔茨海默病 (AD) 的新发病机制。先前在 Aβ40 和 Aβ42 单体中研究了受组氨酸互变异构影响的聚集机制。在本研究中,我们专注于组氨酸影响下 Aβ40 异源二聚体的结构特性。分子动力学模拟结果检测到εεε异构体和其他异构体形成的不同异二聚体存在不同的聚集趋势。在(εεε:δδδ)二聚体中,δδδ链中β-折叠含量最高,这与我们之前的研究一致,即δδδ单体是单体中最容易形成的β-折叠二级结构。进一步的分析证实,与其他异源二聚体相比,(εεε:δδδ) 二聚体更容易聚集成原纤维。











































 京公网安备 11010802027423号
京公网安备 11010802027423号