Kidney International ( IF 14.8 ) Pub Date : 2021-09-14 , DOI: 10.1016/j.kint.2021.08.023 Junnan Xu 1 , Xiubin Li 1 , Qing Yuan 1 , Chenfeng Wang 1 , Liang Xu 1 , Xing Wei 1 , Haitao Liu 1 , Bo Yu 2 , Zhekun An 3 , Yuanyu Zhao 4 , Xiang Li 1 , Xu Zhang 1 , Xin Ma 1 , Ming Cai 5
|
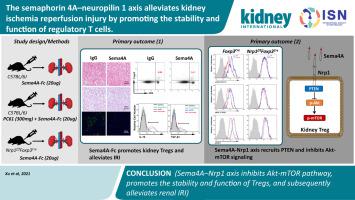
Previous studies have suggested the role of CD4+Foxp3+ regulatory T cells (Tregs) in protection against kidney ischemia reperfusion injury via their immunosuppressive properties. Unfortunately, the associated mechanisms of Tregs in kidney ischemia reperfusion injury have not been fully elucidated. Semaphorin 4A (Sema4A) is essential for maintaining the immunosuppressive capacity of Tregs in tumors. However, whether Sema4A can alleviate kidney ischemia reperfusion injury through Tregs has not yet been demonstrated. Here, we investigated the effect and mechanism of Sema4A on the development of kidney ischemia reperfusion injury. Administration of recombinant human Sema4A-Fc chimera protein prior to ischemia reperfusion injury promoted the expansion and function of Tregs and decreased the accumulation of neutrophils and proinflammatory macrophages thereby attenuating functional and histological injury of the injured kidneys. Depletion of Tregs abrogated the protective effect of Sema4A on kidney ischemia reperfusion injury, suggesting Tregs as the main target cell type for Sema4A in the development of this injury. Mechanistically, Sema4A bound to neuropilin 1 (Nrp1), a cell surface receptor for Sema4A and other ligands and a key regulator of Tregs, which then promoted recruitment of phosphatase and tensin homologue and suppressed the Akt–mTOR pathway in Foxp3Cre mice but not in Nrp1f/f Foxp3Cre mice. Consistently, Treg-specific deletion of Nrp1 blocked the effect of Sema4A on the expansion and function of Treg cells. Thus, our results demonstrate that the Sema4A–Nrp1 axis alleviates the development of ischemia reperfusion injury by promoting the stability and function of Tregs in mouse kidneys.
中文翻译:

semaphorin 4A-neuropilin 1 轴通过促进调节性 T 细胞的稳定性和功能减轻肾脏缺血再灌注损伤
以前的研究表明 CD4 + Foxp3 +的作用调节性 T 细胞 (Tregs) 通过其免疫抑制特性防止肾缺血再灌注损伤。不幸的是,Tregs 在肾脏缺血再灌注损伤中的相关机制尚未完全阐明。Semaphorin 4A (Sema4A) 对于维持肿瘤中 Tregs 的免疫抑制能力至关重要。然而,Sema4A 是否可以通过 Tregs 缓解肾脏缺血再灌注损伤尚未得到证实。在这里,我们研究了 Sema4A 对肾脏缺血再灌注损伤发展的影响和机制。在缺血再灌注损伤之前施用重组人 Sema4A-Fc 嵌合蛋白可促进 Tregs 的扩张和功能,并减少中性粒细胞和促炎巨噬细胞的积累,从而减轻受伤肾脏的功能和组织学损伤。Tregs 的消耗消除了 Sema4A 对肾缺血再灌注损伤的保护作用,表明 Tregs 是 Sema4A 在这种损伤发展中的主要靶细胞类型。从机制上讲,Sema4A 与神经纤毛蛋白 1 (Nrp1) 结合,Nrp1 是 Sema4A 和其他配体的细胞表面受体,也是 Tregs 的关键调节剂,然后促进磷酸酶和张力蛋白同源物的募集并抑制 Akt-mTOR 通路 表明 Tregs 作为 Sema4A 在这种损伤发展中的主要靶细胞类型。从机制上讲,Sema4A 与神经纤毛蛋白 1 (Nrp1) 结合,Nrp1 是 Sema4A 和其他配体的细胞表面受体,也是 Tregs 的关键调节剂,然后促进磷酸酶和张力蛋白同源物的募集并抑制 Akt-mTOR 通路 表明 Tregs 作为 Sema4A 在这种损伤发展中的主要靶细胞类型。从机制上讲,Sema4A 与神经纤毛蛋白 1 (Nrp1) 结合,Nrp1 是 Sema4A 和其他配体的细胞表面受体,也是 Tregs 的关键调节剂,然后促进磷酸酶和张力蛋白同源物的募集并抑制 Akt-mTOR 通路Foxp3 Cre小鼠,但不在Nrp1 f/f Foxp3 Cre小鼠中。一致地,Nrp1 的 Treg 特异性缺失阻断了 Sema4A 对 Treg 细胞扩增和功能的影响。因此,我们的结果表明,Sema4A-Nrp1 轴通过促进小鼠肾脏中 Tregs 的稳定性和功能来减轻缺血再灌注损伤的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号