Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-09-15 , DOI: 10.1016/j.bioorg.2021.105356 Cheng-Peng Sun 1 , Yi-Bo Chang 1 , Chao Wang 1 , Xia Lv 1 , Wei-Yu Zhou 2 , Xiang-Ge Tian 1 , Wen-Yu Zhao 1 , Xiao-Chi Ma 3
|
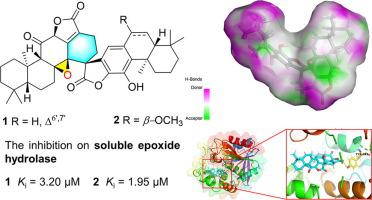
Two undescribed ent-abietane-type diterpenoid dimers with nonacyclic backbone formed by intermolecular [4 + 2] cycloaddition into a spirocyclic skeleton, bisfischoids A (1) and B (2), along with a known one fischdiabietane A (3), were identified from Euphorbia fischeriana Steud. Their structures were elucidated by extensive spectroscopic analysis, ECD and NMR calculation combined with DP4+ probability analysis, as well as X-ray diffraction. The anti-inflammatory potential of dimers 1–3 were examined using their inhibitory effects on soluble epoxide hydrolase (sEH), which revealed that 1 and 2 exhibited promising activities with inhibition constant (Ki) of 3.20 and 1.95 μM, respectively. Further studies of molecular docking and molecular dynamics indicated that amino acid residue Tyr343 in the catalytic cavity of sEH was the key site for their inhibitory function.
中文翻译:

Bisfischoids A 和 B,来自 Euphorbia fischeriana Steud 的具有抗炎潜力的二聚体-松香烷型二萜。
两个未描述的ENT -abietane型二萜类与nonacyclic骨干二聚体通过分子间形成[4 + 2]环加成成螺环骨架,bisfischoids A(1)和B(2),与已知的一个fischdiabietane A(沿3),被确定来自大戟属fischeriana Steud。它们的结构通过广泛的光谱分析、ECD 和 NMR 计算结合 DP4+ 概率分析以及 X 射线衍射来阐明。二聚体的抗炎潜力1 - 3使用它们对可溶性环氧化物水解酶(SEH),其显示抑制效果考察了1和2表现出有希望的活性,抑制常数 ( K i) 分别为 3.20 和 1.95 μM。分子对接和分子动力学的进一步研究表明,sEH催化腔中的氨基酸残基Tyr343是其抑制功能的关键位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号