Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2021-09-15 , DOI: 10.1016/j.bioorg.2021.105361 Chao Liu 1 , Yining Li 1 , Ren Sheng 1 , Xiaowan Han 1 , Li Bao 2 , Chenyin Wang 1 , Weizhi Wang 1 , Xinhai Jiang 1 , Jiangxue Han 1 , Lijuan Lei 1 , Ni Li 1 , Jing Zhang 1 , Minghua Chen 1 , Yan Li 1 , Yexiang Wu 1 , Shunwang Li 1 , Yu Ren 1 , Yanni Xu 1 , Shuyi Si 1
|
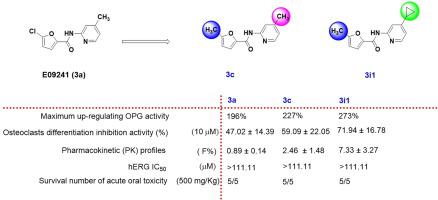
The OPG/RANKL/RANK pathway is a promising target for the design of therapeutic agents used in the treatment of osteoporosis. E09241 with an N-methylpyridine-chlorofuranformamide structural skeleton was previously identified to decrease bone loss and thus protect against osteoporosis in ovariectomized rats through increasing osteoprotegerin (OPG) expression. In this study, 36 derivatives of E09241 (3a) were prepared. The synthesis, up-regulation of OPG activities, SAR (structure–activity relationship), and cytotoxicity of these compounds are presented. Compounds with good up-regulating OPG activities could inhibit RANKL (the receptor activator of nuclear factor-kappa B ligand)-induced osteoclastogenesis in RAW264.7 cells. Particularly, compounds 3c and 3i1 significantly reduced NFATc1 and MMP-9 protein expression through inhibition of the NF-κB and MAPK pathways in RANKL induced RAW264.7 cells. In addition, compounds 3c and 3v significantly promoted osteoblast differentiation in MC3T3-E1 cells in osteogenic medium, and compounds 3c, 3v, and 3i1 obviously increased OPG protein expression and secretion in MC3T3-E1 cells. Furthermore, the pharmacokinetic profiles, acute toxicity, and hERG K+ channel effects of compounds 3a, 3c, 3e, 3v, and 3i1 were investigated. Taken together, these results indicate that N-methylpyridine-chlorofuranformamide analog 3i1 could serve as a promising lead for the development of new agents for treating osteoporosis.
中文翻译:

N-甲基吡啶-氯呋喃甲酰胺类似物作为新型 OPG 上调剂和 RANKL 诱导的破骨细胞生成抑制剂的合成
OPG/RANKL/RANK 通路是设计用于治疗骨质疏松症的治疗剂的有希望的目标。具有N-甲基吡啶-氯呋喃甲酰胺结构骨架的E09241先前已被鉴定可减少骨质流失,从而通过增加骨保护素 (OPG) 表达来防止去卵巢大鼠的骨质疏松症。本研究共制备了 E09241 ( 3a ) 的36 种衍生物。介绍了这些化合物的合成、OPG 活性的上调、SAR(结构-活性关系)和细胞毒性。具有良好上调 OPG 活性的化合物可以抑制 RANKL(核因子-κB 配体的受体激活剂)诱导的 RAW264.7 细胞中的破骨细胞生成。特别是,化合物3c和3i1通过抑制 RANKL 诱导的 RAW264.7 细胞中的 NF-κB 和 MAPK 通路,显着降低了 NFATc1 和 MMP-9 蛋白的表达。此外,化合物3c和3v在成骨培养基中显着促进MC3T3-E1细胞成骨细胞分化,化合物3c、3v和3i1明显增加了MC3T3-E1细胞中OPG蛋白的表达和分泌。此外,化合物3a、3c、3e、3v和3i1的药代动力学特征、急性毒性和 hERG K +通道效应被调查。总之,这些结果表明N-甲基吡啶-氯呋喃甲酰胺类似物3i1可以作为开发治疗骨质疏松症的新药物的有希望的先导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号